FDA Deputy Commissioner of proposed Human Foods Program moves forward with aggressive agenda while waiting for reorg finalization targeted for June
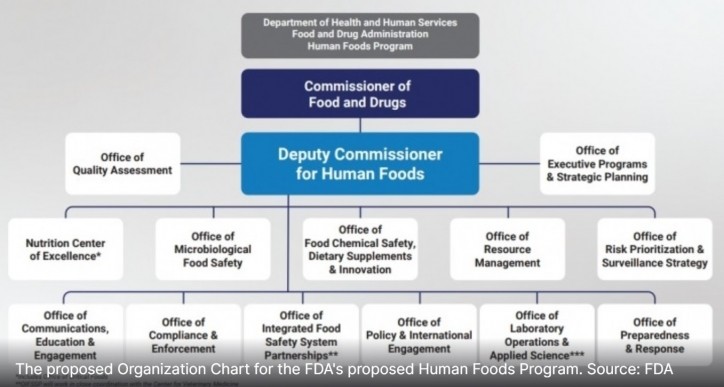
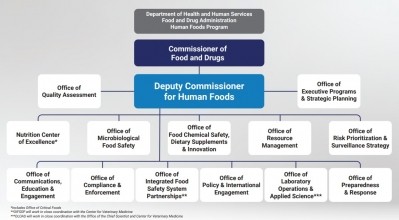
Less than two months after stepping into the role of the first Deputy Commissioner for Human Foods, which will oversee 12 offices and one center and report directly to FDA Commissioner Robert Califf, Jones has already toured farms and met with inspectors and field staff in California, Kansas City and St. Louis and is planning trips to New York, Atlanta and elsewhere in the coming months.
“I’ve committed to … getting out into the field at least once a month. I think it’s really important for my role as a regulator to see things for myself – the impacts that we’re having, but also to be with our field staff,” and work with stakeholders “early and often” so that the new Human Foods Program, when it is finalized, can move forward quickly with “eyes wide open” and “inevitably make better choices,” Jones said yesterday in a webinar hosted by the Alliance for a Stronger FDA.
He added he is also committed to increasing transparency into the Human Foods Programs’ priorities and progress and “letting people have an opportunity to give feedback” before the agency progresses too far or has a chance to get off course and risk needing a do-over.
Taking into account lessons from these meetings, early feedback from stakeholders as well as recommendations from the Reagan-Udall report, which called for the creation of his job, Jones said his team is “getting … really very close to submitting the package to HHS” for what the new Human Foods Program will look like under his leadership.
Human Foods Program could be finalized by early summer
Knowing that it will take time for the Office of Management and Budget as well as the appropriations subcommittees in the House and Senate to review the package, Jones said he thinks “it is reasonable to be targeting June of next year” for finalizing the reorganization.
But, as illustrated by his active engagement in the field, he simultaneously is moving forward in the next six to nine months with “very high priority activities,” including a proposal to enhance safety of agriculture water, proposed revisions to front-of-pack nutrition labeling, finalizing the definition and s potential symbol to represent the claim ‘healthy’ for packaged foods, and articulating the agency’s 2024 chemical safety work plan.
Each of these goals falls into what Jones says will be the Human Foods Program’s initial top three priorities: preventing foodborne illness through the creation of an Office of Microbiological Food Safety, improving nutrition by creating and elevating a Nutrition Center of Excellence and enhancing chemical safety – including the review of food additives – through the Office of Food Chemical Safety, Dietary Supplements and Innovation.
Elevating chemical safety should help FDA ‘get in front’ of state activity, additive bans
Of these three, Jones said he thinks the “most surprising” for many people is the elevation of enhancing chemical safety to the office level, but he argues it is the “only way meaningfully” that FDA can “get in front” of state actions related to food additives and other ingredients, such as California’s recently passed Food Safety Act, which bans the sale and distribution within the state of products containing brominated vegetable oil, potassium bromate, propyl paraben and Red Dye No 3.
He explained that since joining FDA one of the most common questions he hears is what the agency is doing to ensure food chemical safety and address state activity related to additives, which threatens to create a patchwork of regulations with which companies likely will struggle to comply.
“The only way for us to get in front of this issue is to have a more meaningful agenda around food chemical safety, and so we will not only be, in this reorganization standing up an office that has got food chemical safety as its focus, but we will become more ambitious in our post chemical review agenda,” he said.
He added this should have an indirect influence on state activity in the space by showing states that “FDA is taking care of business” so that they can focus on other priorities that benefit their citizens.
‘There is a lot of really meaningful activity in the nutrition space’ for the coming year
Elevating nutrition within FDA with the creation of the Nutrition Center of Excellence also responds to stakeholders’ calls for action, Jones said.
Initial priorities within this office include finalizing voluntary sodium targets, revising regulations related to “healthy” labeling claims, exploring how to reduce added sugar consumption, reducing children’s exposure to contaminants through food through the agency’s Closer to Zero initiative and more.
“There is a lot of really meaningful activity in the nutrition space all planned for the coming year,” Jones said.
Reorg will consolidate partnerships, compliance for food borne illness prevention
Finally, preventing food borne illness is a top motivator for many of the proposed reorganization elements and initial office activities, Jones said.
“The reorganization really is a means towards helping to bring some improvements into our efforts to prevent foodborne illness … and some of the things we are doing in the reorganization, we think, will help to optimize the talent pool we have here,” he said.
For example, he said, the new structure will bring the agency’s laboratories together under one organization and consolidate compliance efforts – both of which will reduce the number and cost of transactions between organizations and speed results.
The new office also will consolidate inspection partnerships with states to better help present foodborne illness, he added.
While there is much to do and many kinks to still work out with the reorganization, Jones concluded that the is “incredibly” excited to work together with stakeholders to “bend the curve in a number of areas,” and he reiterated his desire to hear from stakeholders early and often as the agency pursues change.