Regulatory Reform
Throughout 2018 we continued to reinforce our position that the Food and Drug Administration (FDA) should streamline regulations to keep consumers safe as the demand for natural products continues to grow. In this regard we have secured valuable wins for consumers, including the delay of the FDA’s costly and burdensome nutritional supplement facts labeling rule, saving consumers hundreds of millions of dollars. We have been hard at work with the Administration and submitting official comments on a number of regulatory issues, including NDI draft guidance, probiotic supplement labeling, homeopathic drugs, bioengineered food disclosure standards, skip lot testing for nutritional supplements and a host of other important issues for consumers and our industry.
Tariffs
While we share the Administration’s goal to help American jobs and businesses, we have been clear about our view during public hearings and comments that tariffs could cause significant harm to our industry’s ability to innovate, grow, and meet consumer demand for natural products.
For our members, the key issue is whether raw materials are available in safe supply from other sources, and the fact is simply that they are not. China has and will continue to be THE virtual sole source on the globe that can handle the large-scale demand of finished product manufacturers in the U.S., and this did not come about organically. On the contrary, China’s role in our supply chain is the result of years of collaboration between our two countries, including the development of quality assurance, safety, good manufacturing practices, and regulatory compliance. In short, we have built today’s positive and proven supply chain, and we want to continue being the primary beneficiary of that hard work.
We continue to work with USTR to make these concerns known, and find it promising that we have been able to secure exemptions for key ingredients including glucosamine and sucralose. We remain optimistic that we will eventually be persuasive, allowing us to meet consumer demand as we shift our focus to other top policy goals in 2019.
Opioid Epidemic
As the opioid epidemic continues to wreak havoc across the country, we have been working with the Administration to do our part to mitigate it, and in the case of kratom, prevent it from getting worse. Kratom, a tropical tree native to Southeast Asia has been linked to overdose deaths and that addiction specialists warn could be making the opioid crisis worse. Kratom is banned in Vermont, the District of Columbia and five other states for a reason, and NPA shares the FDA’s concerns that kratom, like any other product, should be subject to the same rules that our industry follows.
CBD
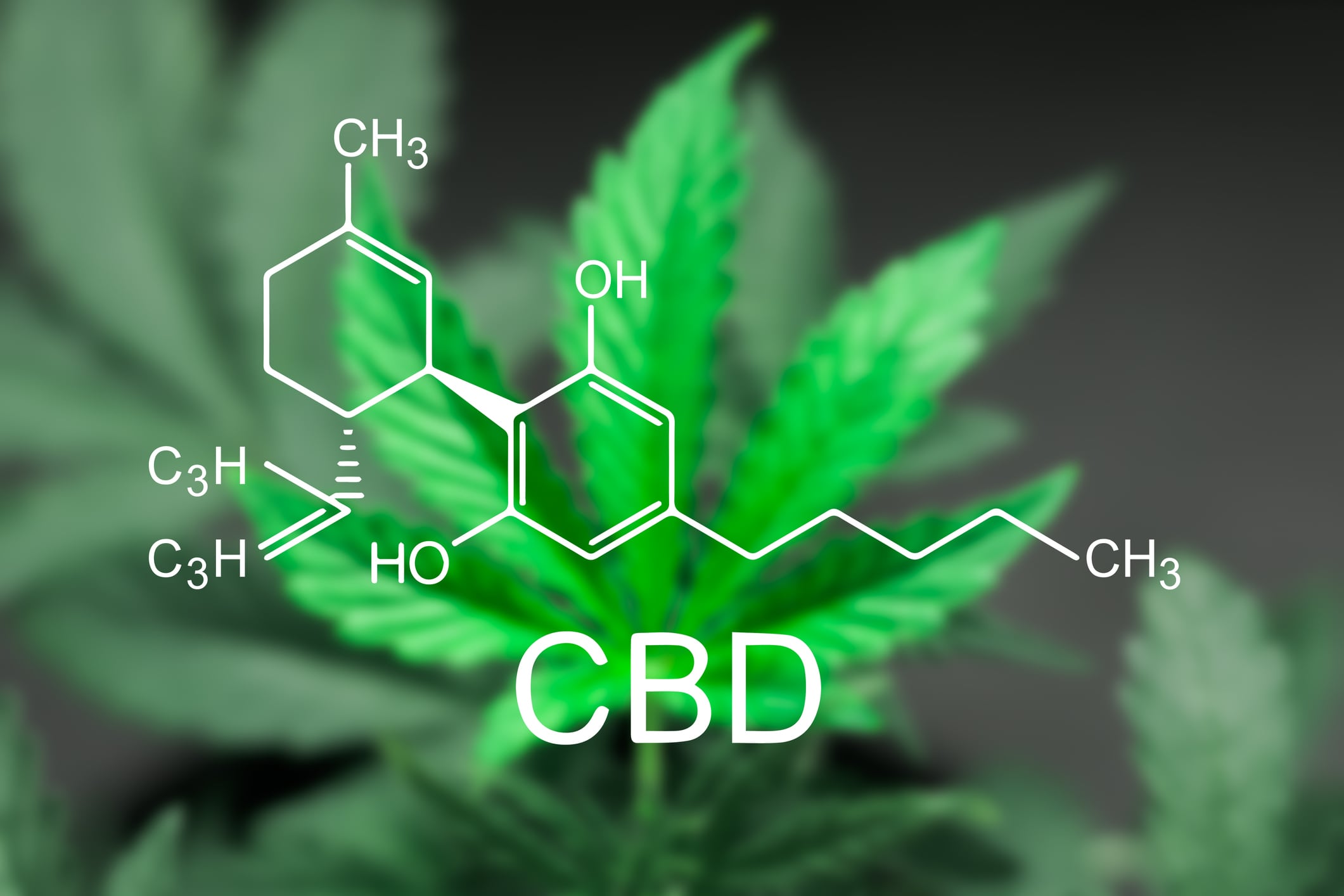
Cannabidiol or CBD is another area that has attracted significant attention throughout 2018 and will continue to draw attention next year. While CBDs may not present the same safety concerns as kratom, there is still a proven path for these products to come to market, as the FDA recently demonstrated with the approval of Epidiolex, a CBD based drug approved to treat epilepsy. Responsible companies must go through the regulatory process, ensuring the products that make it to consumers are proven to be safe and reliable.
Focusing on kratom and CBD is important, because a large part of our industry’s success is due to a strong federal regulatory regime, significant investment in product safety and quality, consumer demand, and self-regulatory programs developed by the men and women of our industry.
Access to Supplements
Another of our leading priorities is continuing to increase nutrition for all Americans by expanding access to nutritional supplements through federal and state social assistance programs and tax incentives. We have long advocated that supplements be included in the Special Supplemental Nutrition Program (SNAP) and the Women, Infants, and Children (WIC) programs. WIC provides 8 million American families the ability to purchase healthy and nutritious foods. With the help of our Members of Congress, we can provide low income women, infants, and children the equal access to vitamin and mineral supplements they deserve.
Likewise, allowing American consumers to use Health Savings Account (HSA) and Flexible Spending Account (FSA) dollars on nutritional and dietary supplements is another innovation that could lead to better nutrition, better health, and potentially fewer hospital visits. NPA is leading the coalition to help pass the Health Savings Act, which makes needed changes to tax laws so that nutritional supplements are treated equally and can qualify for HSA and FSA expenditures.
Advocacy

Washington, DC and state capitals are in constant states of change, and we must stay committed to maintaining our current relationships and begin building new ones. We were proud to host record attendance at this year’s Natural Products Day. We also know that the changing of control in the House of Representatives and the retiring of our long-time Champion Senator Orrin Hatch means that we will have to remain vigilant as we move into 2019. Our core message to policymakers this year was simple: Our industry is safe and strongly-regulated. Americans depend on today’s products and are hungry for more new, natural alternatives in their lives. And they must realize the positive role our industry plays not just for the health of millions of Americans but also the health of the U.S. economy and good, high-paying jobs.
It was another busy year in the natural products industry, and we look forward to engaging in these and other critical public policy debates in the year ahead.

