FDA data: Dietary supplement recalls relatively infrequent

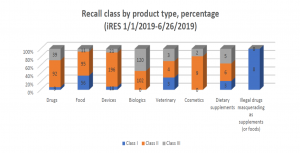
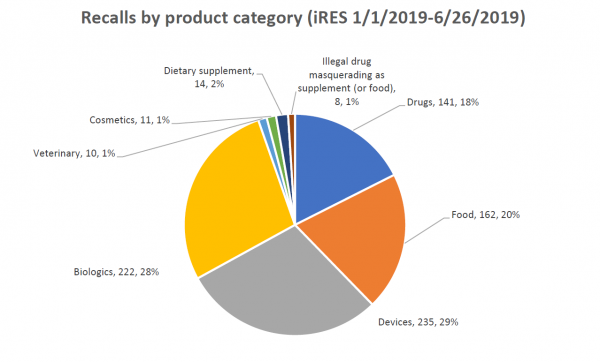
Recall data from the Food and Drug Administration (FDA) show that 14 of 803 (1.7 %) recorded recalls initiated in 2019 involved dietary supplements, and among these, three were Class I recalls (the most serious recall class), according to a report published by American Herbal Products Association (APHA).
FDA assigns voluntary recalls a classification based on a health hazard evaluation:
- Class I -- A reasonable probability that the use of, or exposure to, a violative product will cause serious adverse health consequences or death
- Class II -- Use of, or exposure to, a violative product may cause temporary or medically reversible adverse health consequences or where the probability of serious adverse health consequences is remote
- Class III -- Use of, or exposure to, a violative product is not likely to cause adverse health consequences
Among the dietary supplements recalled between January 1, 2019, and June 26, 2019, four cases were due to risk of microbial contamination, three cases were for mislabeling, and two cases were for the presence of unlabeled allergens. During the same period, 162 food products were recalled; 73 cases were for undeclared allergens or the presence of sulfur or sulfites, 41 cases were related to microbial contamination, 26 cases were for manufacturing errors usually involving foreign material in the product, and 11 recalls were based on mislabeling.
“These recall data provide additional evidence of the overall safety of the dietary supplement class,” said AHPA Chief Information Analyst Merle Zimmermann, Ph.D. “AHPA also regularly reviews other dietary supplement safety resources, including mandatory serious adverse event reports and recorded observations from FDA inspections, and the results suggest that current supplement laws and regulations are working effectively to protect consumer safety and ensure a marketplace of high-quality, safe products.”
The report is the first of its kind from the trade association. APHA’s Director of Communications, Haley Chitty, told NutraIngredients-USA that they plan to continue to add data to the report and build it out over time.