Promotional Features
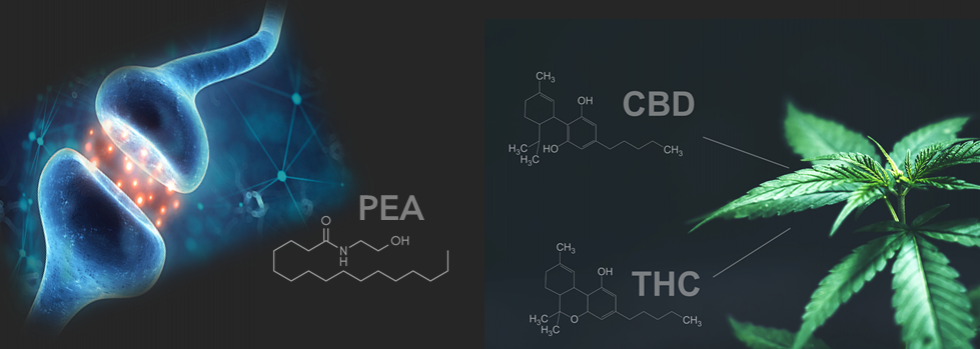
The “Magic” of CBD vs. The “Science” of PEA
Consumers are increasingly looking for the natural alternatives to traditional medicines, and this is one of the main reasons that CBD products have become so popular. Awareness of CBD is at an all-time high, and in a 2021 report from Research and Markets, the global cannabis market is estimated to be valued at USD 20.5 billion in 2020 and is projected to reach USD 90.4 billion by 2026, recording a CAGR of 28%.
However, CBD is facing a list of challenges now that include a lack of human clinical studies, legalities, safety, compliance, dosing, and mislabeled products. Additionally, CBD is increasingly being scrutinized by the European Food Safety Authority (EFSA), the Medicines and Healthcare products Regulatory Agency (MHRA), as well as the U.S. Food and Drug Administration (FDA).
CBD has been shown to have anti-inflammatory, analgesic activity and neuroprotective properties1-6 but has yet to be clinically validated in a healthy population and proven safe as studies have only shown evidence in diseased populations with anecdotal evidence. Without further high-quality studies and evidence with human trials, consumer won’t be able to identify the proper efficacies and doses of the product. Furthermore, due to CBD being readily available as an unregulated supplement, consumers really don’t know what they are buying.
Scientific and safety concerns are only the tipping point with CBD. Many brands are coming under fire for making wild and unsubstantiated based on anecdotal evidence or evidence seen in diseased populations. The FDA continues to maintain its position that CBD cannot be lawfully marketed in dietary supplements or conventional food because, the compound was first studied as a drug. While CBD is no stranger to the shelves of many retail outlets, gas stations, grocery stores and vape shops, many large retailers, the likes of Target and Walmart have remained on the sidelines as they wait to see what the FDA does. The FDA is looking to possibly craft rules for CBD in approved ingestible products but has not yet committed to doing so.7
For consumers, the appeal of the supposed health benefits from CBD outweigh the regulatory concerns. CBD for many is positioned as the “holy grail” of supplements with its proposed ability to benefit stress, inflammation-related discomfort, mood, and immune health. Taking a deeper dive into CBD and its mechanism has revealed that the endocannabinoid system could be the key to unlocking the benefits that consumers are looking for in their supplements.
The Endocannabinoid System or ECS
The ECS is an important system involved in balancing numerous bodily functions including stress, inflammation, mood and immunomodulation. It consists of the endogenous cannabinoids (endocannabinoids), cannabinoid receptors, and enzymes that synthesize and degrade endocannabinoids8. Well know endogenous cannabinoids include anandamide, n-arachidonoyl dopamine, and virodhamine.9 The cannabinoid receptors in the ECS can be found in our skin, immune cells, bones, fat tissue, kidneys, GI tract, liver, and various other places throughout our bodies10. Two receptors, called CB1 and CB2, mediate many effects of these cannabinoids and endocannabinoids within the central nervous system.8,9
CBD and the ECS
When CBD enters the body, it does very little with the endocannabinoid system (ECS). It does however activate or inhibit other compounds or cell receptors within the body. For example, CBD acts upon cannabinoid receptors CB1 and CB2 to limit the breakdown of the compound anandamide (AEA)11 or the “bliss molecule” that is associated with regulating discomfort. It is important to note that AEA is an endogenous cannabinoid known to act upon CB1 receptors in the skin to reduce discomfort.11,12 When needed, our bodies naturally create AEA on-demand and is then used immediately. Fatty acid amide hydrolase (FAAH), then comes and breaks down AEA, and it has been shown that CBD may inhibit FAAH before it can breakdown the AEA.11
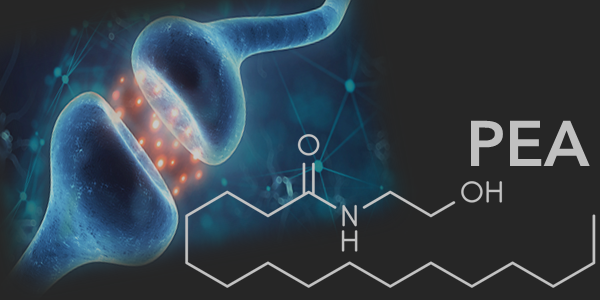
Another compound that has shown to have an effect on AEA is Palmitoylethanolamide (PEA). PEA is an endogenous fatty acid amide produced naturally in the body in response to injury and stress. It is found in lipid extracts of foods and plants such as egg yolk, peanuts and soybeans, as well as produced naturally in the body.
PEA and the ECS
Recent research has shown that PEA, similar to CBD, can influence the endocannabinoid system. Unlike CBD, PEA is structurally related to the bliss molecule, and because of this, it may co-enhance AEAs effects as well as inhibit FAAH.13 PEA also works both directly and indirectly within the central and peripheral nervous systems. Directly, PEA reduces inflammation locally by halting the activity of pro-inflammatory genes and the production of many inflammatory substances via PPAR-α receptors.14 Indirectly, PEA enhances the levels and actions of other compounds (e.g. AEA) that are anti-inflammatory and provide analgesic relief. This mechanism is known as the ‘entourage effect’.14 AEA’s ability to bind on to both CB receptors and TRPV-1 channels helps combat inflammation and increase relaxation.
PEA resembles CBD in that both offer anti-inflammatory and neuro-protective properties. However, CBD is not produced by the human body unlike PEA, which is endogenously produced as a direct response and repair mechanism to inflammation. PEA counteracts endotoxin-induced inflammation in cells in the same cell lineage as CBD.
The PEA Difference
PEA has been substantiated by more than 40 studies for different benefits, thereby making it a safe and clinically proven alternative to CBD.15,16
In three studies on sleep, PEA was shown to reduce anxiety and improve quality of life providing PEA’s relevance and application in sleep.17 As part of the extended endocannabinoid system, PEA promotes restful sleep through indirect activation of the calming CB1 receptors.18 PEA can increase REM sleep by activating TRPV1 channels.14 PEA has also shown to optimize the immune system.16 This is due to PEA’s unique actions on numerous pathways, such as activation of PPAR-a and inhibiting mast cell degranulation.18
A New Kind of PEA
Because of its fatty nature, PEA has trouble dissolving in water which causes a drastic reduction with both bioavailability and absorption inside the body’s digestive system. To overcome the challenge, Gencor utilized the novel, award-winning LipiSperse delivery technology, created by Pharmako Biotechnologies to develop a branded form of PEA named Levagen+, which significantly increases both the bioavailability and functionality of PEA.
One pharmacokinetic study showed that LipiSperse technology showed an approximate 1.8x increase in PEA blood levels when comparing a 300mg dose of Levagen+ to a 300mg dose of the standard PEA formulation.19
In a recently published study in the International Journal of Nutrition and Food Sciences, the study assessed the efficacy of dispersible Palmitoylethanolamide – PEA (Levagen+) for alleviating joint discomfort and improving quality of life in adults.
The randomized, double-blind study compared the efficacy of a 350 mg dose of Levagen+ PEA to placebo on relieving joint discomfort in healthy adults aged between 25 and 70 years. Participants in the PEA group consumed 175 mg of Levagen+ twice daily (morning and night) with water over a two-week period.
Both groups demonstrated an early reduction in VAS (visual analog scale) pain scores compared to baseline values until approximately day 10 when the scores plateaued and increased in the placebo group while continuing to decrease in the PEA group, demonstrating the efficacy of PEA at reducing joint discomfort compared to placebo over the 14-day study period. The study also validated the bioavailability effects of dispersible PEA (Levagen+) vs. standard PEA by utilizing a lower dose (350 mg) compared to 600 mg dose of standard PEA that was used in previous trials to study PEA’s benefits for joint health.20
With increased bioavailability and its ability to disperse freely in water, Levagen+ can be used in a variety of delivery formats beyond capsules and tablets. These innovative formats include: RTD shots, effervescents, gels, gummies, powders, and beverages.
It is important to note that PEA naturally decrease with age, and when an individual encounters painful and/or inflammatory stimuli (e.g. exercise-induced inflammation), PEA levels drop quickly as they start binding on to PPAR-a receptors. Exogenous supplementation is therefore needed in order to restore levels of PEA in the body.
We don’t need banish CBD to the basement and label it “The Boogie Man” – CBD very well may be beneficial it is just too early to be conclusive as many of the claims aren’t backed by clinical research in a healthy population. With so many questions about long-term side effects, and regulatory mountains to be conquered, brand manufacturers should consider a safe and clinically validated CBD alternative – Levagen+ PEA.
Levagen+ PEA has been studied to provided targeted benefits for joint health, sports recovery, inflammation, relaxation and sleep.
Levagen+ PEA is GRAS, approved by Health Canada as an NHP, and accepted as a dietary supplement in U.S., Europe, Australia and India.
Visit LevagenPlus.com to learn more >>
-----
References:
1 Hartsel JA, Eades J, Hickory B, Makriyannis A.Cannabis sativa and Hemp. Nutraceuticals: Efficacy, Safety and Toxicity, pp. 735–754, 2016.2 Appendino G, Chianese G, Taglialatela-Scafati O.Cannabinoids: Occurrence and medicinal chemistry. Curr Med Chem vol. 18, no. 7, pp. 1085–1099, 20113 Fern.ndez-Ruiz J, Moreno-Martet M, Rodr.guez-Cueto C et al. Prospects for cannabinoid therapies in basal ganglia disorders. Br J Pharmacol vol. 163, no.7, pp. 1365–1378, 2011.4 Alexander SPH. Therapeutic potential of cannabisrelated drugs. Prog Neuro- Psychopharmacol & Biol Psychiatr vol. 64, pp. 157–166, 2016.5 Campos AC, Foga.a MV, Sonego AB, Guimar.es FS. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol Res, vol. 112, pp.119–127, 2016.6 Brenneisen R. Chemistry and analysis of phytocannabinoids and other Cannabis constituents. In Marijuana and the Cannabinoids, El Sohly MA, Ed., 49, 17 pages, Humana Press Inc., Totowa, NJ, USA, 2007.7 Falling Star? – CBD industry faces new raw reality - https://store.newhope.com/collections/issues/products/august-2020-the-finance-issue8 Mackie, K. (2008, May). Cannabinoid receptors: where they are and what they do. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/18426493.9 CBD and the Endocannabinoid System: Learn More. (n.d.). Retrieved from https://cbdoilreview.org/cbd-cannabidiol/cbd-endocannabinoid-system/10 Learn About Cannabis. (n.d.). Retrieved from https://www.uclahealth.org/cannabis/learn-about-cannabis11 “[EGuide] Endocannabinoid: The Body’s Master System.” Delicious Living, 28 Nov. 2018, www. deliciousliving.com/health/endocannabinoid-thebodys-master-system/.12 De Filippis D, Luongo L, Cipriano M, Palazzo E, Cinelli MP, de Novellis V, Maione S, Iuvone T. Palmitoylethanolamide reduces granuloma-induced hyperalgesia by modulation of mast cell activation in rats. Mol Pain. 2011 Jan 10;7:3.13 Ho WS, Barrett DA, Randall MD. ‘Entourage’ effects of Npalmitoylethanolamide and N-oleoylethanolamide on vasorelaxation to anandamide occur through TRPV1 receptors. Br J Pharmacol. 2008 Nov;155(6):837-46.14 Petrosino, S., & Marzo, V. D. (2016, September 29). BPS Publications. Retrieved from https:// bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/ bph.13580.10.1111/(ISSN)1476-5381.nutraceuticalsjoint-virtual-issue.15 Hesselink, J.M.K. (2012). New targets in pain, nonneuronal cells, and the role of palmitoylethanolamide. The Open Pain Journal, 5, 12-23.16 Hesselink J.M.K., et al. (2013). Palmitoylethanolamide: a natural body-own anti-inflammatory agent, effective and safe against influenza and common cold. International Journal of Inflammation. In press.17 Steels, E., Venkatesh, R., Steels, E. et al. A double-blind randomized placebo controlled study assessing safety, tolerability and efficacy of palmitoylethanolamide for symptoms of knee osteoarthritis. Inflammopharmacol 27, 475–485 (2019). https://doi.org/10.1007/s10787-019-00582-918 Petrosino, S., and Di Marzo, V. (2017) The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. British Journal of Pharmacology, 174: 1349– 1365. doi: 10.1111/bph.13580.19 Briskey, D., A. R. Mallard, and A. Rao. "Increased Absorption of Palmitoylethanolamide Using a Novel Dispersion Technology System (LipiSperse®)." J Nutraceuticals Food Sci 5.2 (2020): 3.20 David Briskey, Georgia Roche, Amanda Rao. The Effect of a Dispersible Palmitoylethanolamide (Levagen+) Compared to a Placebo for Reducing Joint Pain in an Adult Population – A Randomised, Double-Blind Study. International Journal of Nutrition and Food Sciences. Vol. 10, No. 1, 2021, pp. 9-13. doi: 10.11648/j.ijnfs.20211001.12Disclaimer:
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.