Promotional Features
NMN New Clinical Finding: Obvious NAD(H) Level Increase
With 7.3% CAGR, The "Nicotinamide Mononucleotide (NMN) Market" size is expected to grow from 194.2 million USD In 2020, to reach 296.5 Million USD by 2026. We can see a large potential market for this cellular ingredient in the dietary supplements field. Meanwhile, we’re encountering many challenges such as strict regulations in many countries, lacking innovative formulations for novel fields etc.
New study
On 5th May 2022, Frontier in Aging, an authoritative international journal on aging mechanisms, published one NMN human clinical trial report showing good efficacy and safety in middle aged and older adults. The 66-subject trial built on evidence that NMN has no acute and sub-chronic toxicity and is not mutagenic and clastogenic.
In this analysis, compared with the placebo group, the blood cellular NAD(H), 6 minute walking endurance test, SF-36 in the NMN group showed a trend of improvement. As an endogenous compound, NMN cannot be used as a drug to rejuvenate the human body in a short time, but the experiment has shown the improvement trend of NMN.
Highlights of this trial
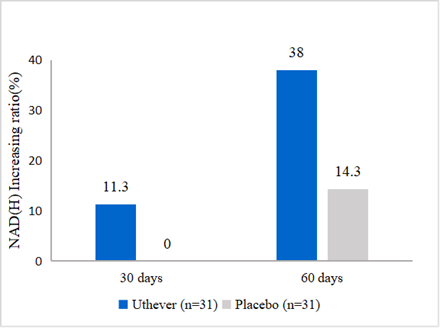
- NMN increases NAD(H) level by 38%
NMN is a precursor to a vital molecule for energy production and metabolic health called nicotinamide adenine dinucleotide (NAD+). Some human studies were trying to evaluate the efficacy of NMN to increase NAD(H) and test NMN metabolites. But the results cannot be shown visually.
In this report, at the end of the study (day 60), the NAD(H) levels were increased further by 38% from baseline in the NMN group, compared to a 14.3% rise in the placebo group.
Figure1
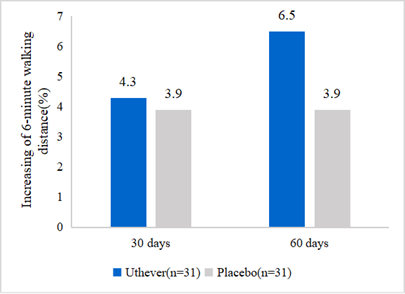
- NMN upgrades walk endurance
NMN is widely applied in anti-aging supplements, but hardly found in sports nutrition. Effepharm tested the walk endurance of NMN for the first time. The walking endurance increased by 6.5% in the NMN group and 3.9% in the placebo group on day 60 of the treatment. From this analysis, it was clear that the placebo effect was evident until day 30, but after that, the NMN group showed further improvement in walking endurance, whereas the placebo group remained at the same level.
Figure2
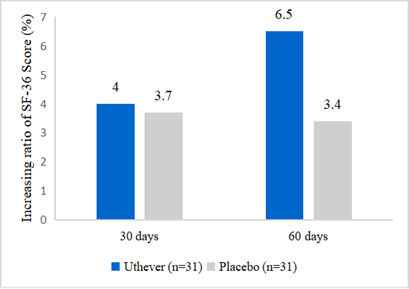
- Subjects of NMN in this study feel happier
Effepharm used the SF 36 questionnaire to demonstrate the rise of subjects’ health scores. At day 60, the NMN group showed a rise of 6.5%, whereas the placebo group was raised by 3.4%. The increase in scores in the NMN group was almost double the increase seen in the placebo group.
Figure 3
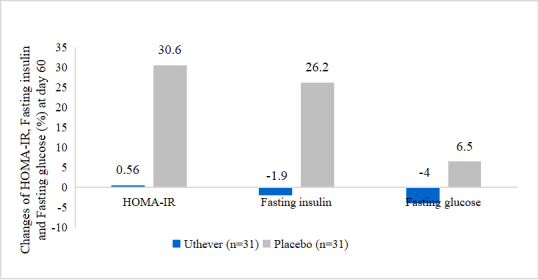
- NMN Improves the stability of glucose and serum insulin
The little changes of HOMA-IR and fasting insulin and fasting glucose in the NMN group indicated the more stability of glucose and serum insulin.
At the end of the study, the mean HOMA IR index showed a rise of 0.6% among the NMN group and a rise of 30.6% among the placebo group from baseline. Mean glucose (sugar) fasting showed a fall of 4.0% among the NMN group and rise of 6.5% among the placebo group from baseline. Mean serum insulin fasting showed a fall of 1.9% among the NMN group versus a rise of 26.2% among the placebo group from baseline.
Figure 4
Introducing UTHEVER
Effepharm launched a branded NMN ingredient to bring the purest and safest NMN ingredient around the world. UTHEVER NMN has been clinically proven to be safe and to improve the NAD(H) level in the human body, thus realizing the anti-aging function.
Effepharm ran the clinical trials to establish a more professional and scientific image, helping the downstream supplement brand side get more power to do product endorsement and give more confidence to end consumers. Supplement brands such as Prohealth Longevity in the US and Do Not Age in the UK are already using the UTHEVER trademark and realizing the co-branding effects.
UTHEVER Partner
More possibility of UTHEVER NMN
On 29th April 2022, Cell Discovery, an authoritative international journal on molecular and cell biology, published one report in treatment of COVID-19 mouse models, with new insights being brought into the field of coronavirus precaution and treatment. Effepharm supplied NMN to support this study looking for more possibilities on NMN application. NMN supplementation can protect 30% of aged mice infected with the lethal mouse-adapted SARS-CoV-2 from death. Dr. Yu also revealed that the first period of NMN clinical trials mainly aimed to verify the safety of NMN, so we designed a lower dosage to some extent. In the next few years, we will consider conducting more NMN function research to explore the safety of larger doses of NMN and the effectiveness of related indications on which this trial data are based. Effepharm has been applying NDI and novel food on NMN, which means that NMN raw material through the food regulations of various countries has taken a milestone step.
Reference:
[1] https://www.frontiersin.org/articles/10.3389/fragi.2022.851698/full
[2] https://www.nature.com/articles/s41421-022-00409-y