Promotional Features
Fenugreek galactomannans as a natural alternative to conventional OTC heartburn medication
Gastroesophageal reflux disease (GERD) is one of the most prevalent gastrointestinal conditions in the Western population and responsible for 7m visits to health facilities in the United States in 2010 (1). Prevalence worldwide ranged from 2.5% in China to 51.2% in Greece, and is probably around 10-20% of the population (2). GERD is characterized by a chronic retrograde flow of gastric content to the esophagus or even the respiratory tract. It can cause a variety of clinical manifestations both esophageal and extra-esophageal, such as heartburn, acid regurgitation, chronic cough and asthma-like symptoms; most of these resulting in significant impairment in patients’ health-related quality of life.
Advancing age increases the prevalence of GERD. Other risk factors are a higher body mass index, smoking and the consumption of alcohol, coffee, and carbonated drinks (3).
The most common therapy for the treatment of GERD today involves pharmacological interventions like antacids, proton pump inhibitors (PPIs), histamine‐2‐receptor antagonists (H2RAs), which make up the most of the market share, followed by a smaller share of alternatives such as sucralfate, prokinetic drugs, and alginate‐based raft formulations (4).
Antacids hold the dominant market share as preferred as first-line therapy for mild and infrequent symptoms because of their convenient over-the-counter availability in various forms such as tablets, powders, and syrups (4).
Antacids are made of alkaline agents that neutralize the gastric acid, resulting in temporary symptomatic relief (5). H2RAs and PPIs have a different mode of action and both interfere with gastric acid production (6). PPIs accounted for the second largest segment as they are the most commonly prescribed medication type for heartburn (4). Despite their ubiquity and wide availability, PPIs can have severe side effects. The long- term consequences of chronic PPI use include the potential increased risk of calcium and magnesium malabsorption, Clostridium difficile infections, and pneumonia. PPIs may be linked to significant medical problems such as heart attacks, kidney disease, dementia, and bone fractures (7).
H2RAs work by blocking histamine and thus decreasing the amount of acid released by cells of the stomach.
Gastric acid plays a vital role in digestion by denaturing proteins and facilitating the absorption of essential nutrients such as folic acid, ascorbic acid, β‐carotene, and increasing the solubility and bioavailability of various minerals. The acidic pH of gastric acid prevents fungal or bacterial infections in the small intestine by making the environment unfavorable for growth. The presence of gastric acid is thus essential for normal physiological functions of the body.
The goal is to deal with acid reflux without interfering with the natural defense barriers and digestive function of the body (3).The best way to manage GERD is thus to limit the contact between the esophagus and the mixture of acid and food from the stomach, rather than disrupting the acid balance.
Since it was first discovered in September 2019 that there were low levels of a probable carcinogen, N-nitrosodimethylamine (NDMA), found in both brand name and generic forms of ranitidine, the U S Food and Drug Administration is investigating whether this poses a risk to patients (8). Ranitidine is an H2RA that works by blocking histamine and thus decreasing the amount of acid released by cells of the stomach.
The FDA has advised companies to recall their ranitidine if testing shows levels of NDMA above the acceptable daily intake, and several ranitidine products already have been voluntarily recalled and removed from the shelves.
Patients taking prescription ranitidine who wish to stop should talk to their health care professional about other treatment options. Consumers taking over-the-counter (OTC) ranitidine may consider using other OTC products approved for their condition. This will spur consumers to look for natural and safe alternatives for acid reflux.
Fenulife - a natural solution
FenuLife is a unique source of deodorized galactomannan fiber produced from fenugreek (Trigonella foenum-graecum). Fenugreek has been traditionally used for various medicinal applications and more recently its wider benefits have been researched in greater depth. Most of the action of FenuLife can be attributed to its molecular structure.
Fenugreek gum has the typical galactomannan structure of 1→4 linked β-D-mannosyl backbone with single unit galactose side chains, α-linked at the O-6 oxygen.
The ratio of galactose to mannose for fenugreek gum is 1:1 when compared to guar gum and locust bean gum having ratio of 1:2 and 1:4 respectively. The lack of free mannoses makes fenugreek galactomannans indigestible by human enzymes, and prevents the formation of fiber crosslinks. Fenugreek gum containing more galactose has superior solubility and dispersiveness and forms stable colloid for a long time compared to other galactomannans.
Milk formulas enriched with water-soluble fibers are already a first-line measure for infants with gastroesophageal reflux. (9).
A clinical study suggests that FenuLife may provide for relief of symptoms often associated with stomach acid reflux (10).
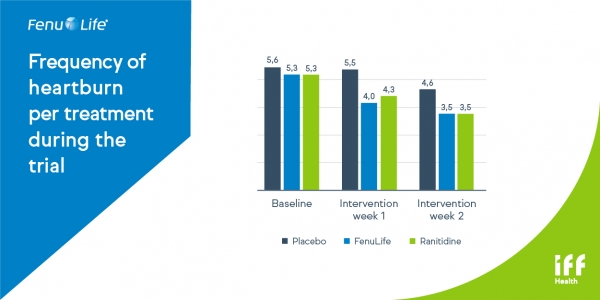
In a two-week placebo-controlled, randomized clinical trial, subjects suffering from heartburn, on average of 3-8 times per week, were randomly assigned to receive FenuLife , placebo or ranitidine for 2 weeks after a 1 week baseline period for the pre-treatment characterization of heartburn symptoms. Only placebo and FenuLife groups were blinded to treatment.
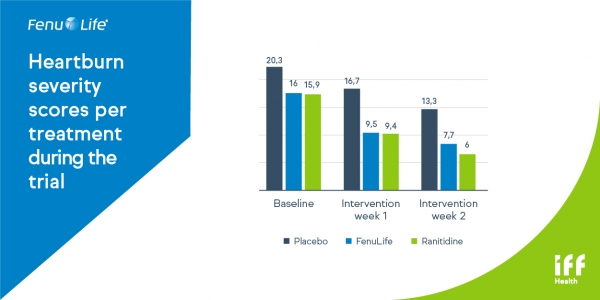
All subjects were instructed to take the assigned product 30 minutes before their two biggest meals of the day with the other meal to be a bland meal. Self-reported heartburn symptoms were used to assess activity of each treatment group. After two weeks of FenuLife intake, subjects reported less severe heartburn symptoms and fewer days with symptoms as compared to their symptoms before FenuLife intake began. The results reported with FenuLife were similar to those seen with ranitidine treatment, although subjects in the placebo group also experienced a reduction in symptoms after 2 weeks. However, the placebo effect was not as great as for the treatment with FenuLife or positive control.
Fig 1 .Total score per week for heartburn severity of each heartburn episode decreased significantly in both FenuLife and control group versus placebo (p<0,05)
Fig 2. Total days with heartburn decreased during the 2 weeks of the trial (p<0,05 versus placebo and >0,05 versus control).
This study indicates that FenuLife may provide relief in subjects experiencing symptoms of acid reflux, although further research is warranted.
Fenulife - mechanism of action
The fenugreek galactomannan produces its activity by mechanical actions without affecting gastric functions and stomach pH. It is believed to bind the stomach acid and food, preventing it to be regurgitated, and protecting the gastric and esophageal lining.
Previous clinical studies studied the effects of FenuLife in weight management through actions to affect the feeling of satiety and to affect the glycaemic index.
In one clinical study, obese subjects had 0, 4 g or 8 g of FenuLife added to breakfast foods and were asked to report levels of satiety. Each subject was given the three breakfast foods on different days (blinded to the amount of FenuLife in the food). Objective measures of activity monitored included blood glucose and insulin levels immediately after food intake which was compared to their baseline levels. Individuals experienced higher levels of satiety and fullness and reduced hunger and prospective food consumption only after ingestion of the 8 g FenuLife . There were no significant effects on glycemic index, however. The effects on appetite and food intake suggest that FenuLife may be useful in weight management and slimming formulations for use in obesity (11).
Galactomannans are known to balance sugar absorption in the small intestine, thereby decreasing spikes in blood sugar following a meal. Thus, another study was designed to investigate if the intake of fenugreek galactomannans influences serum glucose in type-II diabetic patients.
Japanese type-II diabetic patients were divided into 2 groups according to their blood glucose levels at baseline (12). One group of people with fasting serum glucose levels of 120-200 mg/dl and a second group with blood sugar levels > 200 mg/dl, who were allowed to continue anti-diabetic drug treatment during the test period of 8 weeks, were given FenuLife (2 g/day) and placebo (lactose) capsules 30 minutes before each meal.
Fasting blood glucose levels were measured at baseline and at 4 and 8 weeks. FenuLife reduced serum glucose levels in both groups of subjects.
FenuLife contains 85% dietary fiber content which can act as prebiotic when incorporated as a functional food. Because they are not metabolized by the enzymes in the human system, fenugreek galactomannans act as dietary food fibers and can promote the colonization of beneficial colon bacteria. It has been shown that fenugreek galactomannans can be fermented in the intestine (by Bacillus coagulans), and thus can serve as a prebiotic (13).
Beside its biologic activity as a fiber source, FenuLife can be incorporated into food products as a food stabilizer, adhesive and emulsifying agent. It can be used for the development of healthy and nutritious extruded and bakery products (14). Fenugreek gum has textural, thickening, emulsifying, stabilizing, gelling and encapsulating properties. It can be used as a soluble dietary fiber for incorporation into nutrition and dairy products, cereal bars, yogurts and nutritional beverages. Bakery foods such as bread, pizza, cakes and muffins and extruded snacks have been prepared by using flour fortified with eight to ten percent soluble dietary fiber improving nutritional value and resulting in a lowered GI index (15).
The fenugreek seeds are milled and the fiber is enriched by a water extraction without the use of solvents, which makes it a suitable ingredient for clean-label formulations.
Consumers are likely to look for a natural, clean label alternative to support their digestive health. Clinical studies suggest that FenuLife may assist in reducing symptoms of acid reflux.
In addition to incorporation of the ingredient into dietary supplement products, there is the potential for use of fenugreek galactomannans in fortified foods to support wellbeing.
References
1. Peery AF, et al., Burden of Gastrointestinal, Liver, and Pancreatic Diseases in the United States. Gastroenterology. 2015;149:1731-41.e3. 2015
2. Zaterka S, et al., Historical perspective of gastroesophageal reflux disease clinical treatment. Arq Gastroenterol. 13;56(2):202-208. 2019
3. Yousaf M et al., Raft‐forming polysaccharides for the treatment of gastroesophageal reflux disease (GORD): Systematic review. 2019
4. Gastroesophageal Reflux Disease (GERD) Therapeutics Market Analysis By Drug Type (Antacids, Proton Pump Inhibitors, H2 Receptor Blocker, Pro-Kinetic Agents), By Region, And Segment Forecasts, 2018-2025
5. Hershcovici T et al., The lower esophageal sphincter.Neurogastroenterol. Motil. 23(9), 819. 2011
6. Galdo J A, Long-term consequences of chronic proton pump inhibitor use. US Pharm. 2013, 38(12), 38. 2013
7. Yan X et al, Estimates of all-cause mortality and cause specific mortality associated with proton pump inhibitors among US veterans: cohort study, BMJ. 2019
8. https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-and-press-announcements-ndma-zantac-ranitidine
9. Fabiani E et al., Effect of a water-soluble fiber (galactomannans)-enriched formula on gastric emptying time of regurgitating infants evaluated using an ultrasound technique.
J Pediatr Gastroenterol Nutr. Sep;31(3):248-50. 2000
10. Disilvestro et al., Anti-heartburn effects of a fenugreek fiber product. Phytotherapy Research 25(1): 88-91. 2010
11. Mathern JR et al., Effect of fenugreek fiber on satiety, blood glucose and insulin response and energy intake in obese subjects. Phytotherapy Research 23(11): 1543-1548. 2009
12. Abe H et al., Effect of FenuLife® on blood glucose in type-II diabetes, unpublished results. 2002
13. Majeed M et al., Galactomannan from Trigonella foenum-graecum L. seed: Prebiotic application and its fermentation by the probiotic Bacillus coagulans strain MTCC 5856. Food Sci Nutr. 20;6(3):666-673. 2018
14. Wani SA and Kumar P. Fenugreek: A review on its nutraceutical properties and utilization in various food products. Journal of the Saudi Society of Agricultural Sciences. 2018; 17 (2) : 97-106. 2018
15. Ravindran G et al., A comparative study of the effects of three galactomannans on the functionality of extruded pea–rice blends. Food Chem. 124, 1620–1626. 2011