Promotional Features
ENOTprost: The new ingredient for prostate food supplements
A new extract of Epilobium angustifolium L. standardized to contain 15% Oenothein B. The right ingredient for male wellness
Prostatic ailments are among the most common urologic problems in adult males. BPH (benign prostatic hyperplasia) is the third most frequent urologic diagnosis in men over 50, although prostatitis can also affect young people, especially sportsmen and cyclists.
Prostatic ailments share inflammation and oxidative stress as risk factors and cause of chronicity.
ENOTprost is a dry extract with a very rich phytocomplex containing more than 15% of Oenothein B.
It is a strong anti-inflammatory and antioxidant ingredient for innovative food supplements.
Epilobium angustifolium L. (Onagraceae) is a well-known European plant traditionally used for prostatic ailments and micturition disorders.
It contains three major polyphenolic families: flavonoids, phenolic acids and ellagitannins.
Flavonoids include both flavanol aglycones (myricetin, quercetin, kaempferol) and flavonoid glycosides (hyperoside, isoquercetin, quercitrin and miquelianin).
Miquelianin (myricitin-3-O-glucorinide) is the major flavonoid glycoside from E. angustifolium and has been detected only in this species; it is also shown in the Enotprost metabolic profile.
Phenolic acids include chlorogenic, gallic, cinnamic, caffeic, ferulic acids, while ellagitannins are mainly represented by the macrocyclic ellagitannin Oenothein B.
No monograph on Epilobium angustifolium L. is available in the Eur. Ph. 9th Ed.; the dry powdered extract is obtained by hydroalcoholic extraction and standardized to contain ≥15% of Oenothein B (HPLC method validated in EPO Srl R&D laboratory).
The extraction method was optimized through DOE (Design of Experiment or experimental design) based on a statistical approach by doing many factorial experiments, ensuring result validity, reliability and replicability with appropriate levels of statistical power and sensitivity.
Oenothein B properties
Although the mechanism of action is not fully understood, Oenothein B could be partially responsible for the Epilobium extract bioactivities.
Oenothein B has immunomodulatory, antioxidant and anti-inflammatory properties; the combined enhancement of innate immune defences and protection of host tissues through antioxidant effects could allow Oenothein B to optimally provide health benefits.
Thanks to the collaboration with the University of Pavia, EPO Srl carried out a preliminary study to assess bio accessibility and bioactivity (anti-inflammatory and antioxidant) using aerial parts of the plant (“herba”), collected in eastern Europe at spring time and then dried.
The botanical species is certified by DNA barcoding analysis.
Proven activity
Bio accessibility is the quantity of compounds released from the matrix (in this case from the powdered dry extract) in the gastrointestinal tract, becoming available for absorption (e.g. enters the bloodstream).
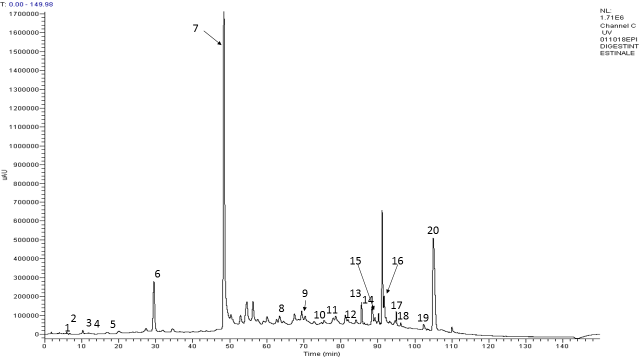
The metabolic profile of ENOTprost was studied after oral-gastro-duodenal digestion and duodenal digestion, simulating, in this latter case, the gastro-resistant oral administration.
According to the results, oro-gastro-duodenal digestion notably reduces the number of bioactive molecules, including Oenothein B, while duodenal digestion maintains the phytocomplex variety and chemical profile as shown in the chromatogram.
The digested extract was rich in 20 compounds among which organic acids, hydroxycinnamic acids, many flavonols and the most represented one, i.e. the ellagitannin Oenothein B.
Miquelianin is the major flavonoid glycoside that characterizes the E. angustifolium species (peak 19).
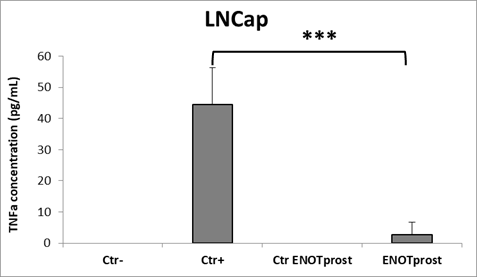
The anti-inflammatory effect was tested using the LPS-induced inflammation model.
Monocyte cells (THP-1 cell line) were inflamed by LPS treatment and induced to produce pro-inflammatory cytokines.
In parallel, to test the anti-inflammatory activity of ENOTprost, prostatatic LNCaP cells were pretreated with Enotprost and then incubated with the cytokines previously produced by THP-1 cells simulating the inflammation process.
Using ELISA test, tumour necrosis factor-α (TNF-α) was measured in LNCaP cells. This molecule is a key cytokine that influences inflammation response and metabolism.
The results confirmed that pre-treatment with ENOTprost strongly reduces TNF-alpha compared to control cells as shown in the following histogram.
Legend: Ctrl-: not inflamed and not treated with ENOTprost; Ctrl+: inflamed but not treated with ENOTprost; Ctrl ENOTprost: not inflamed but treated with ENOTprost; ENOTprost: inflamed and treated with ENOTprost
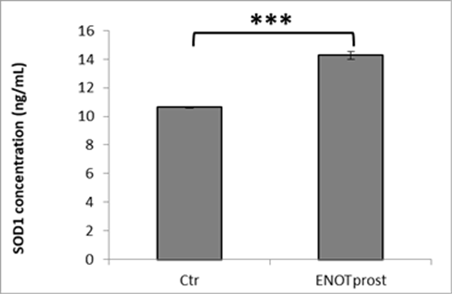
The antioxidant activity was also tested by studying the cellular antioxidant capacity through SOD1 analysis after ENOTprost pre-treatment. This enzyme catalyzes the dismutation (or partitioning) of the superoxide radical into either ordinary molecular oxygen or hydrogen peroxide, which is less toxic than oxigen and eliminated by other cellular enzymes.
ENOTprost was able to enhance cellular antioxidant defences by increasing the level of SOD1 compared to control cells as shown in the following histogram.
Safety and warnings
Health risks or adverse events as well as drug interaction following the proper administration of the drug have not been recorded so far; no concerns arise from the few available data on toxicity.
The use in pregnancy and lactation is not applicable due to the indication. Safety in children and adolescents has not been established: in the absence of enough data, the use of the extract is not recommended.
The recommended intake is 300-400 mg dose 1-2 times daily.
Applications
Food supplements for seniors, sport food supplements and functional foods (bars).
References:
Plants for a future (PFAF) database, www.pfaf.org
Gruenwald J. et al. PDR for herbal medicines, 4th Ed. Thomson, 2007
Andrew Chevallier, Enciclopedia delle piante medicinali, Dorling Kindersley Ed., 1997
Capasso F, Grandolini G, Izzo AA., Fitoterapia, Springer-Verlag Italia, 2006
Assessment report on Epilobium angustifolium L. and/or Epilobium parviflorum Schreb., herba, EMA, 10 March 2015
Schepetkin IA et al. Therapeutic potential of polyphenols from Epilobium angustifolium (Fireweed). Phytother Res. 30(8):1287-97, Aug 2016
Kaškonienė V, Stankevičius M, Drevinskas T, Akuneca I, Kaškonas P, Bimbiraitė-Survilienė K, Maruška A, Ragažinskienė O, Kornyšova O, Briedis V, Ugenskienė R. Evaluation of phytochemical composition of fresh and dried raw material of introduced Chamerion angustifolium L. using chromatographic, spectrophotometric and chemometric techniques. Phytochemistry 115:184-93, 2015.
Granica S, Piwowarski JP, Czerwińska ME, Kiss AK. Phytochemistry, pharmacology and traditional uses of different Epilobium species (Onagraceae): A review. Journal of Ethnopharmacology, 156, 316–346, 2014
Carbonell‐Capella JM, Buniowska M, Barba FJ, Esteve MJ, Frígola A. Analytical Methods for Determining Bioavailability and Bioaccessibility of Bioactive Compounds from Fruits and Vegetables: A Review. Comprehensive review in food science and food safety. 13 (2): 155-171, 2014