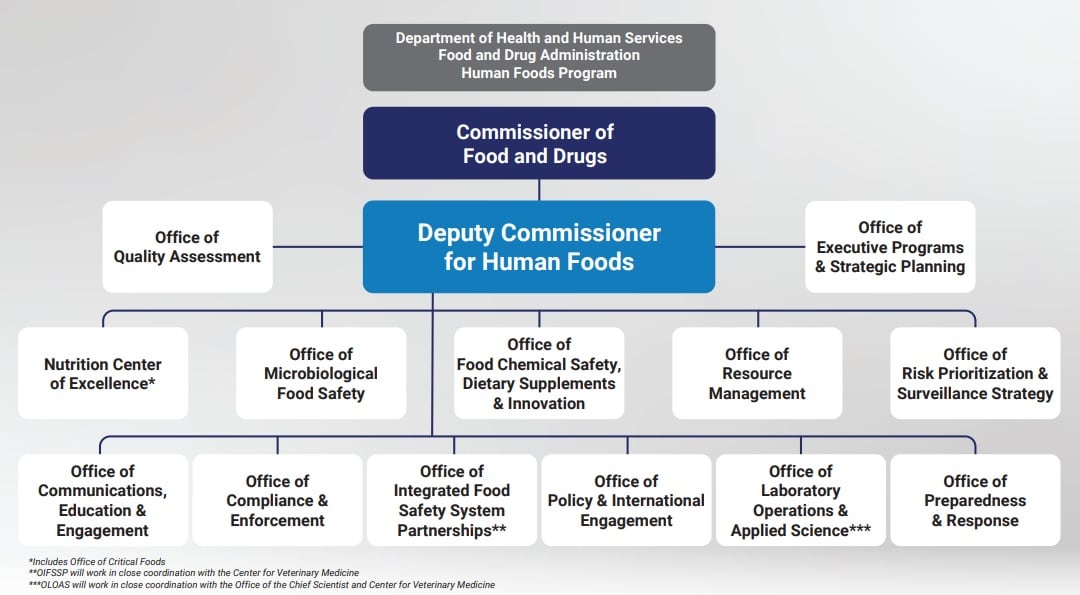
Earlier this summer, the US Food & Drug Administration announced proposals to reorganize its Human Foods Program (HFP) and Office of Regulatory Affairs (ORA), which the Agency hailed as a “transformative vision” for a unified Human Foods Program.
The dramatic overhaul was in response to a scathing report by the Reagan-Udall Foundation that found that the agency’s current culture, structure and governance inhibits its ability to protect public health and to a separate internal review of the agency’s infant formula supply chain response.
As part of the reorganization, FDA is proposing to relocate ODSP within a new Office of Food Chemical Safety, Dietary Supplements, and Innovation.
While Sens. Durbin and Blumenthal welcomed the proposal for the new Deputy Commissioner for Human Foods to have authorities over “all budget and resource allocations for the HFP, including ORA resources”, they expressed concern about the changes to ODSP.
“In 1994, the Dietary Supplement Health and Education Act (DSHEA) established a framework to regulate dietary supplements in the United States. It included provisions to outline the ingredients that could—and could not—be included in a supplement. DSHEA also provided FDA with authorities to oversee the market to ensure that consumers would have access to safe supplements. However, oversight and enforcement at FDA has not kept pace, while the market has seen significant growth over the past 29 years," wrote the Senators.
“ODSP is the lead office at FDA responsible for oversight of the $50 billion supplement market in the United States. With a little more than $13 million in funding, it sets strategic priorities and ensures that limited resources are used in the best manner possible to protect the health and well-being of consumers.
“According to the June 27, 2023, announcement, ODSP will be merged into the 'Office of Food Chemical Safety, Dietary Supplements, and Innovation'. We are concerned that these changes could divert resources, funding, and attention from the supplement market at a time when it is needed more than ever,” they added.
A full copy of the letter can be found HERE.
Burning questions
The Senators are requesting answers to the following questions:
- How will the proposed changes lead to greater oversight and enforcement of the supplement market?
- How many full-time equivalents (FTEs) in the Office of Food Chemical Safety, Dietary Supplements, and Innovation will support the regulation of the supplement market?
- Who will set the goals, priorities, and budget for FDA’s dietary supplement activities?
- Will FTEs be dedicated to one market or split their time between the regulation of the supplement market and other markets (i.e., food chemical safety, innovation)?
- If FTEs split their time, how will this result in greater oversight and enforcement of the supplement market?
- Does FDA plan to hire additional FTEs to support the regulation of the supplement market?
- What are the metrics that FDA will use to evaluate the effectiveness of the Office of Food Chemical Safety, Dietary Supplements, and Innovation, with regard to regulation of the supplement market?
- In Fiscal Year 2023, ODSP received $13.1 million in funding to oversee the $50 billion supplement market. In the future, will FDA request that the House and Senate Appropriations Committees provide dedicated funding for the regulation of the supplement market or general funding for the Office of Food Chemical Safety, Dietary Supplements, and Innovation?
Durbin, who also serves as the Senate Majority Whip, and Blumenthal requested that FDA respond to their questions by September 8, 2023.
NPA: “FDA’s race to reorganize has been done in a tone-deaf manner”
Responding to the new letter from Sens. Durbin and Blumenthal, Daniel Fabricant, PhD, president and CEO of the Natural Products Association (NPA), told NutraIngredients-USA: “When you have both congressional industry champions (like Jeff Duncan) and congressional industry critics saying that effectively demoting the supplement programs into another office versus having a free standing dietary supplement office with greater transparency (which was asked for only a few years ago) doesn’t seem to be the right move for a number of reasons - we hope that gets FDA's attention to listen and keep the office as a standalone. Maybe even to realize that some of their race to reorganize has been done in a tone-deaf manner, and better engagement with stakeholders is needed to ensure accountability going forward.”
Industry opposition
FDA’s proposal has already been met with concern by the dietary supplements industry itself, with major trade associations expressing opposition to the proposal. Speaking with NutraIngredients-USA shortly after the Agency first announced the proposal, Loren Israelsen, President of the United Natural Products Alliance (UNPA) and one of the key architects of DSHEA, described the proposal as “disturbing”.
Israelsen told us that his first response was, “a wave of nausea, and that is because the number two priority in DSHEA is dietary supplements are not food additives - full stop. Congress agreed and that is one of the Ten Commandments of DSHEA, and to see those two terms next to each other under the same office, I found disturbing.”
Steve Mister, President and CEO of the Council for Responsible Nutrition (CRN), told us in July: “We’re very concerned about this reorganization of FDA that would take dietary supplements and combine our office with another office on food chemical safety. That is not a good sign for how FDA looks at dietary supplements and the future of regulation of supplements.”
Michael McGuffin, President of the American Herbal Products Association (AHPA), previously told us the organization’s board met in early July and decided it will communicate to the FDA and Congressional offices opposition to any reorganization of FDA’s Human Food program that subsumes the Office of Dietary Supplement Programs.
“It seems dismissive of the really good progress that [ODSP] has made over the last few years in building its staffing, in building its expertise, in building its relationships, and now do we have to create all of that over again?” he said.
“We like the status quo, and we’ll continue to support the status quo.”