Eric Schneiderman, NY’s Attorney General, commissioned Dr James Schulte II of Clarkson University in Potsdam, NY to perform the DNA barcoding of herbal supplements purchased from the four retailers in 13 regions across NY State. The data has not been made publicly available. (Barcodes are short genetic markers or signatures in DNA that identify which particular species it belongs to.)
“The DNA test results seem to confirm long-standing questions about the herbal supplement industry,” said Schneiderman in a press release. “Mislabeling, contamination, and false advertising are illegal. They also pose unacceptable risks to New York families—especially those with allergies to hidden ingredients. At the end of the day, American corporations must step up to the plate and ensure that their customers are getting what they pay for, especially when it involves promises of good health.”
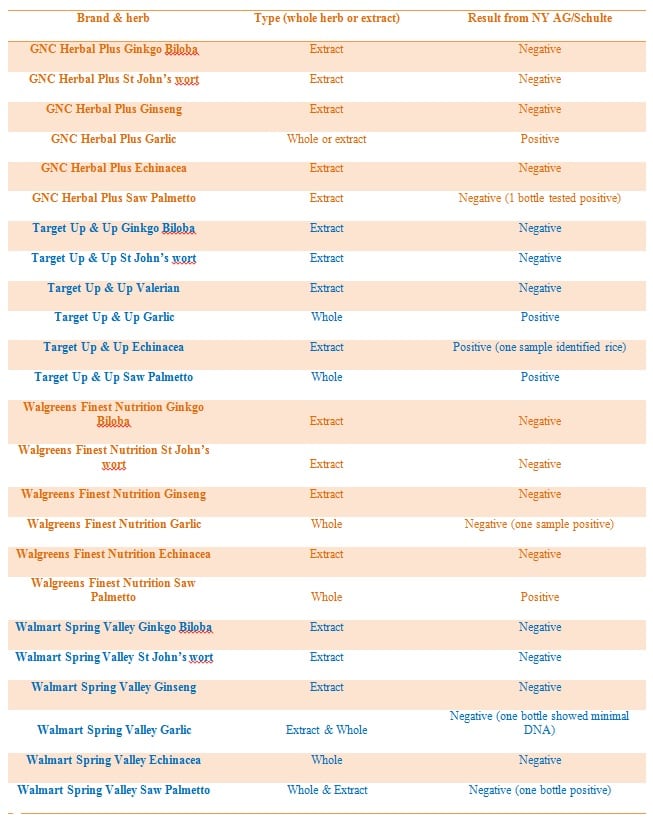
The labels
The AG’s investigation was prompted by a report in the New York Times in 2013 of a controversial paper by Prof Steven Newmaster and his group at the Guelph-based Biodiversity Institute of Ontario in the open access journal BMC Medicine. That paper was widely criticized by analytical and botanical experts and the American Botanical Council called for it to be retracted.
Dr Schulte and the AG did not seem dissuaded by such limitations, however, and used the technique exclusively to analyze samples of Ginkgo Biloba, Ginseng, Valerian, garlic, Echinacea, saw palmetto, and St John’s wort
As reported by NutraIngredients-USA in 2013, DNA barcoding offers a lot of potential for botanical testing, and is incredibly reliable, but only when performed on appropriate material.
Label data for the products tested are not available, but, according to the descriptions from the NY AG’s office and data available from the retailer websites, we estimate that 66% of the products tested were extracts, with a further 8% available as both whole herbs and extracts. That’s a potential 74% of products tested being extracts (see table below for more information. Caution: These numbers are based on products listed on the websites of the four retailers and limited details provided by the AG in a press release. This data has not been confirmed).
Furthermore, the Natural Products Association has confirmed with GNC that every product tested was a botanical extract.
But…
Danica Harbaugh Reynaud, PhD, a geneticist, botanical taxonomist, and CEO of AuthenTechnologies and expert in applying DNA analysis to botanicals, explained that botanical extracts are not appropriate for DNA barcoding. Dr Harbaugh Reynold also questioned the lab’s expertise in this area.
“I’ve never even heard of this lab,” she told us. “They seem to be applying an inappropriate DNA method to finished products. The problem is that the media and the Attorney General will take DNA as the end-all even if it’s not applied properly.
“We have developed a method to analyze extracts but it’s very specific. General DNA barcoding does not work.”
“DNA testing is an emerging technology that has the potential to be useful in the future when it has been rigorously tested and validated – the usual course with new analytical methodologies,” said Michael McGuffin, president of the American Herbal Products Association. “But until DNA testing can stand on its own, any identification of an herb with this method must be confirmed with one or another of the long-established analytical tools that herbal experts use, such as chromatography, microscopy, or organolepsis.”
NutraIngredients-USA contacted Dr Schulte for comment on a number of issues, including his suitability to test botanicals and botanical extracts, but he passed the questions to the NY AG’s office. The NY AG’s office has not responded to requests for comment from this publication.
ABC
Mark Blumenthal, founder and executive director of the American Botanical Council, told us that his organization provided a comment to the New York Times explaining the limitations of DNA barcoding for testing the identity of botanical dietary supplements, and called the results from the one laboratory “preliminary and requiring further substantiation”. [The NYT did not include ABC’s thoughtful comments in its coverage]
“Although some extracts may contain DNA, it is often of low quality or degraded to a point that makes it impossible to perform proper authentication," said Stefan Gafner, PhD, ABC’s Chief Science Officer. "Other processes, like extensive heat treatment, or submission of plant material to UV light also impact the quality and may lead to erroneous results using DNA methods.”
Mark Blumenthal called the NY AG’s actions “problematic”. “We here at ABC are sympathetic with the AG’s obvious concern about the reported problems associated with adulteration of herbal ingredients in dietary supplements, but the AG should take every precaution to approach potential remedies appropriately and responsibly,” he told us.
“Conducting DNA tests with little or no apparent controls, and without employing a second or even a third analytical laboratory to confirm the findings of the first lab results, and then proceeding to engage in regulatory activity (cease-and-desist letters) is premature and unwarranted based on the evidence they have collected thus far. Additional testing in another DNA lab as well as by other appropriate and validated testing methods is required.”
The high failure rates are not seen by other analytical experts. Elan Sudberg, CEO of Alkemist Labs, told us that, for the industry as a whole, his lab does not see failure rates anywhere near the ones listed by the AG.
Questions
There is general frustration among industry stakeholders at the lack of transparency over the data. “There are so many details absent,” said Daniel Fabricant, PhD, CEO of the NPA. “There’s no peer-review. We don’t know the labels of the products. What were the reference materials used? We want to see the full study.”
We have questions of our own...
Please click HEREto read our article: 10 questions we need answered regarding the NY AG’s botanical action
“The federal government and the FDA have never been shy, particularly in New York, about using the full force of the law,” said Dr Fabricant. “I was a federal regulator and I know that any actions become a matter of public record. There is none of that here from the AG. He’s fighting an uphill battle."
Steve Mister, CEO of the Council for Responsible Nutrition, said his organization was “stunned that the AG would use this type of testing and ignore that GMPs requires 100% identity testing.
“These are very reputable brands with mainstream products and a history of passing GMP inspections,” said Mister. “They have rigorous internal and third party inspections and the FDA is routinely in these facilities.”
Expect an aggressive response from the industry, he added. “We need to call out the attorney general to give us the test data and justify why they used DNA barcoding when it’s not a validated method for finished products. We need to put the AG under the same scrutiny as he has put the industry.”
UNPA: ‘The implications are not insignificant’
Loren Israelsen, president, United Natural Products Alliance, said that his organization is currently in consultation with a number of its Science & Technology Services members and appropriate partner organizations “because there has been an ongoing interest in and concerns regarding the viability of DNA testing for botanicals”.
“We will be actively evaluating the data, assuming that it becomes available, to determine the validity of the study and the AG’s actions. Additionally, we will continue to research and review this situation as the implications for responsible industry as well as the public’s perception of it and its products are not insignificant.”
CRN: ‘A self-serving publicity stunt’
CRN’s Mister described the NY AG’s actions as a “self-serving publicity stunt under the guise of protecting public health.
Retailer response
According to the New York Times, Walgreens is removing the products from its shelves nationwide, while Walmart said it is contacting its supplement suppliers “and will take appropriate action.” GNC said it would work with the AG but stood by its products. Target hasn't commented.
“Supposed concerns about the products in question are based on a novel testing method that has been roundly criticized by botanical scientists who question whether DNA barcoding technology is an appropriate or validated test for determining the presence of herbal ingredients in finished botanical products.”
Qualitative vs quantitative
The presence of potential ‘contaminants’, including include rice, beans, pine, citrus, asparagus, primrose, wheat, houseplant, and wild carrot is cited by the AG as posing “unacceptable risks to New York families—especially those with allergies to hidden ingredients”.
However, a bigger question relates to the qualitative versus quantitative aspect of DNA barcoding – the technique is so accurate and sensitive that trace amounts of a foreign material will show up (qualitative) without providing any information about how much of that foreign material is present (quantitative).
“The DNA testing method does not provide information on the amounts of food contaminants found in the products,” said CRN’s Mister. “This is important because there are well-established legal thresholds that allow for trace amounts of some ingredients like gluten, and trace amounts of DNA from rice, beans, pine, citrus, etc. are not considered harmful or required on labels.
“It could be that there is a bit of contamination that is not sufficient to trigger the labeling requirements for allergens, but is enough to show up in the DNA barcoding, but without seeing the data, we don’t know. This is a very one-sided conversation.”
The industry has also criticized how the Attorney General used his data. “Instead of giving companies a reasonable opportunity to respond to these concerns, the AG unfortunately chose to label them guilty without a fair trial,” said Mister. “Not only is the testing method itself suspect for these kinds of products, but the scientist who developed the assay and conducted the testing is not a botanical or a food expert. He is an evolutionary biologist who specializes in testing DNA in dinosaurs and lizards.”
The case is being handled by Executive Deputy Attorney General Marty Mack and Assistant Attorney General Deanna Nelson with the assistance of NYAG’s thirteen regional offices.

