DNA barcoding technology has rarely been far from the headlines since New York Attorney General Eric Schneiderman used it to build cases against a number of retailers of herbal supplements last year.
As reported by NutraIngredients-USA in 2013, DNA barcoding offers a lot of potential for botanical testing, and is incredibly reliable, but only when performed on appropriate material. Industry experts claimed, however, that botanical extracts are problematic because, while some extracts may contain DNA, it is often of low quality or degraded to a point that makes it impossible to perform proper authentication.
In response to questions from NutraIngredients-USA about the suitability of the technology for analysis of botanical extracts, a spokesperson for the Office of the Attorney General told us in February 2015: “Rather than attacking testing methods that have been validated by more than 70 published papers, the time has come for the herbal supplements industry to put concerns about what is and is not included in their products to rest.”
New data from separate studies from the US Food and Drug Administration and an Australian research consortium presented at the prestigious 16th Annual Oxford International Conference on the Science of Botanicals at the University of Mississippi this week supports the long-held industry position.
ABC: “Interested parties should take careful note of these studies”
Commenting independently on the findings presented at Oxford, Mark Blumenthal, founder & executive director of the American Botanical Council, told us: “The research on the use of DNA barcode analytical methodology on finished herbal dietary supplements by the US FDA and on finished supplements and raw botanical materials by researchers in Australia clearly confirm what many botanical analytical experts have been saying all along: that the results of DNA testing methods by themselves are inadequate and inappropriate criteria for accurately and definitively determining the true identity of botanical dietary ingredients, whether as an ingredient alone as well as that ingredient in a matrix of a finished dietary supplement product.
“Regulators, plaintiffs’ attorneys, journalists, industry members, independent laboratories, and other interested parties should take careful note of these studies so that they do not attempt to base any activities simply on the basis of DNA-based analytical methods alone. As most qualified experts have noted, a combination of appropriate analytical methods is necessary for the accurate analysis of botanical materials, particularly those used in dietary supplements.”
FDA data
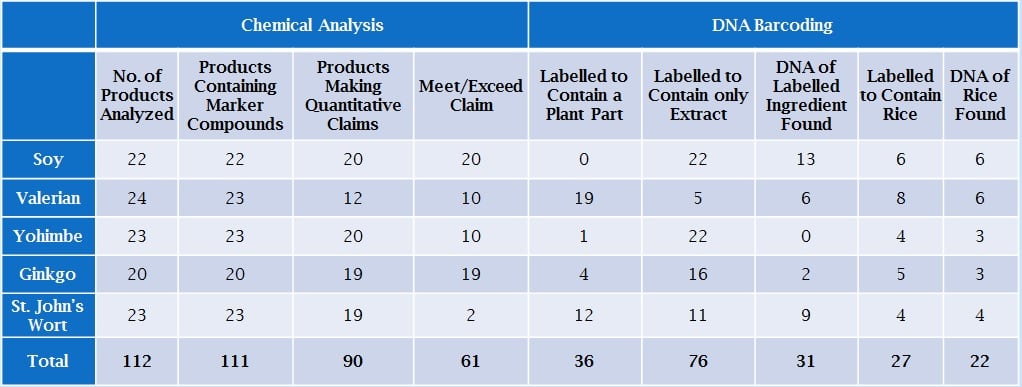
The FDA data was presented by Rahul Pawar, PhD, research coordinator for dietary supplements at CFSAN / FDA’s Office of Regulatory Science. Dr Pawar and his co-workers compared results from HPLC analysis and traditional DNA barcoding of five popular botanical ingredients: Soy, Valerian, Yohimbe, St John’s wort, and Ginkgo.
A total of 112 products were analyzed using both techniques. The data indicated that 111 of these contained marker compounds, according to the HPLC analysis. In addition, of the 90 products that made quantitative label claims, 68% appeared to meet or exceed the label claim, according to the HPLC analysis, said Dr Pawar.
On the other hand, when the products were tested by DNA barcoding, only 31 of the 112 products were found to contain the DNA of the labeled ingredient. Thirty-six products were designated to contain a plant part, and 76 were designated as botanical extracts. The data is presented in the table below.
The Agency has not taken an official position on the application of the technology and the presentation represented the views of Dr Pawar and not the FDA.
A spokesperson for the Agency confirmed that the study’s findings will be submitted for publication in a peer-review journal.
“Entirely unsuitable for extracts and solid dosage forms containing extracts”
Similar conclusions were reached by a team of scientists from Integria Healthcare, The University of Queensland, Southern Cross University, Western Sydney University, and the Australian Genome Research Facility.
Hans Wohlmuth, PhD, R&D Manager for Integria Healthcare, presented the data in Oxford, and told us, “In our study, DNA barcoding proved to be entirely unsuitable for extracts and solid dosage forms containing extracts.”
The study examined the validity and utility of DNA barcoding in the quality control of botanical raw materials, extracts and solid dosage forms.
Study materials
The Australian study tested 15 Herbarium voucher materials, 17 dried herb raw materials, 17 MediHerb liquid extracts, 6 MediHerb tablets containing dried MediHerb liquid extracts, and 6 other products.
MediHerb is an Integria Healthcare brand, and is a leading natural products company that specializes in research-based botanical medicines designed by and for professional health care providers and clinicians.
MediHerb was the recipient of this year’s American Botanical Council Varro E. Tyler Award for Phytomedicinal Research.
“Using batches of authentic raw materials, we followed the genetic barcoding sequences as well as the phytochemical profiles through the manufacturing process, from raw material to finished product,” explained Dr Wohlmuth. “Using four of the most widely used barcoding sequences, we found that DNA barcoding was not able to identify what species most extracts and tablets were derived from.”
On the other hand, liquid chromatography with UV and mass detection clearly demonstrated that extracts and tablets contained the phytochemical profile of the raw materials, including the active compounds, said Dr Wohlmuth. “Even for the raw materials, the barcoding results were disappointing, with only just over half of the samples being successfully identified by at least one of the four barcodes,” he said.
“Based on our findings, we do not believe DNA barcoding with these universal barcode sequences would make a valuable contribution to our existing routine quality control program, which is based on morphological and chemical tests prescribed by pharmacopoeial monographs. This is not to say that other, more species specific DNA tests won't have a role to play in some cases,” Dr Wohlmuth told us.
“Our study highlights the fact that no single method has all the answers when it comes to the complex task of authenticating botanicals. I have no doubt that DNA technology has a contribution to make, but the idea that DNA barcoding can replace all other tests is clearly misguided,” he said.

