Urolithin A, a natural polyphenol metabolite, has emerged as a front-runner in anti-aging research due to its unique ability to regulate cellular metabolism.
As a high-purity urolithin A producer adhering to pharmaceutical standards, Bonerge Lifescience prioritizes evidence-based innovation. Following its landmark oral cosmeceutical trial in 2025, Bonerge has initiated a second randomized, quadruple-blind clinical study targeting sleep quality in middle-aged and elderly populations.
This trial investigates urolithin A’s potential to address fragmented sleep and circadian disruption while validating its holistic anti-aging benefits through multidimensional biomarker monitoring.
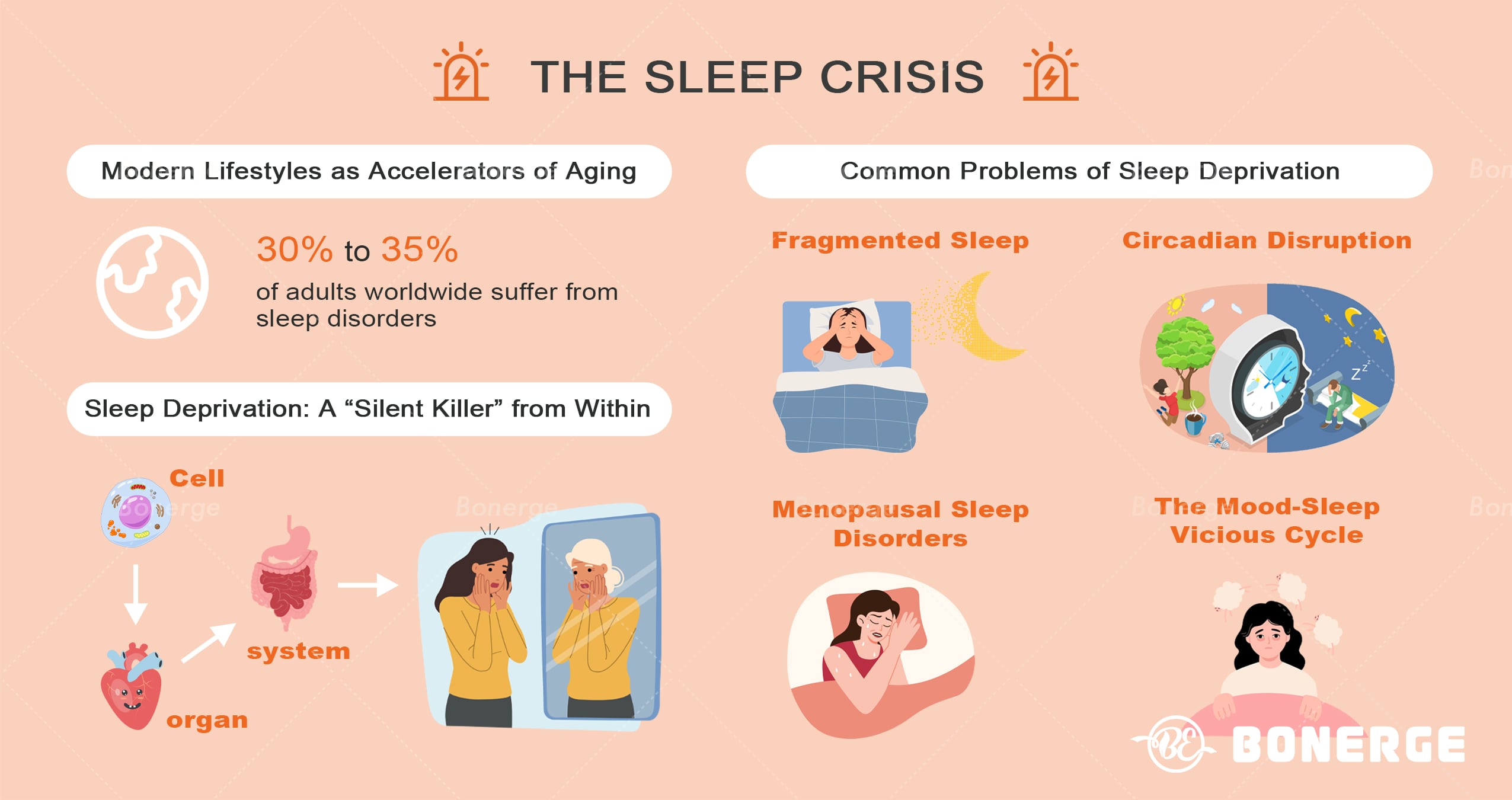
The sleep crisis: Modern lifestyles as accelerators of aging
Globally, 30%-35% of adults suffer from sleep disorders.¹ In the context of globalization and the digital wave, lifestyles characterized by late-night work, fragmented entertainment, and reversed day-night rhythms have become commonplace. The resulting sleep deprivation is accelerating aging process at cellular, organ, and systemic levels, acting as a ‘silent killer’.
1. Fragmented sleep: From disruption to cellular damage
Fragmented sleep, a common type of sleep disorder, refers to interrupted and frequent awakenings during sleep due to exogenous and endogenous factors, leading to light and non-continuous sleep.² Research indicates that almost 66% of surveyed individuals experience fragmented sleep, with the highest prevalence (71.95%) occurring in the 35-44 age group.³ The harm of fragmented sleep extends far beyond mere fatigue.
- Cellular metabolic dysfunction Sleep disruptions impair muscle protein synthesis, elevate fatty acid oxidation and reactive oxygen species (ROS) production in cardiac tissue, and trigger oxidative stress in cells, like liver cells, which might contribute to hepatic injury.⁴
- Senescent cells accumulation Chronic sleep loss upregulates the senescence marker p16INK4a in murine aortic walls and nearly doubles pro-inflammatory IL-6 levels, hastening inflammatory responses and tissue deterioration.⁵
- Metabolic syndrome susceptibility Fragmented sleep exacerbates insulin resistance and hyperglycemia, forming a self-perpetuating cycle with visceral fat deposition.⁴
2. Circadian disruption: When artificial light overrides biology
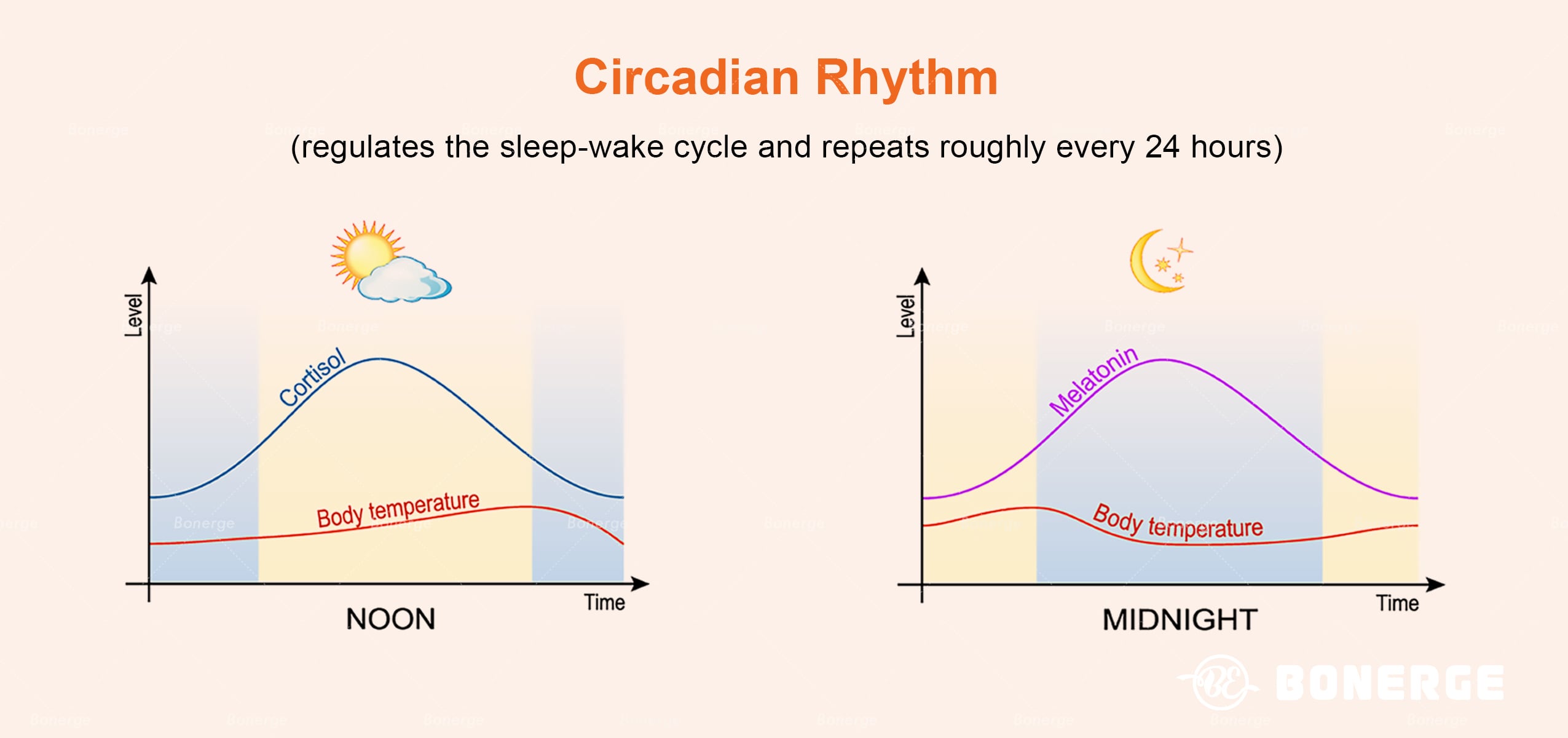
Modern lighting environments (e.g. blue light exposure) and irregular schedules are systematically disrupting circadian rhythms. The circadian clock – a central mechanism regulating sleep-wake cycles – orchestrates 24-hour oscillations in gene expression throughout the body via the suprachiasmatic nucleus (SCN).⁶ Research reveals profound and far-reaching health consequences of circadian disruption:
- Clock gene dysregulation Shift workers exhibit ~2.5-3 hour delays in the rhythmicity of core clock genes PER1 and BMAL1, which further disturbs melatonin secretion patterns.⁷
- Exacerbated inflammation Circadian misalignment enhances Th17 cell activation and elevates IL-17A levels – key drivers of inflammatory and autoimmune disorders such as psoriasis and rheumatoid arthritis.⁸
- NAD+ metabolic disruption NAD+ (nicotinamide adenine dinucleotide), a vital coenzyme for cellular metabolism and energy homeostasis, exhibits circadian fluctuations regulated by clock genes. The NAD+ dependent deacetylase SIRT1 is a major metabolic regulator in this pathway. Circadian disruption significantly reduces peak NAD+ levels, severely impairing cellular energy homeostasis.⁹
3. Menopausal sleep disorders: The ‘multi-hit effect’ of estrogen withdrawal
Sleep disturbances commonly begin during the menopausal transition and become more prevalent after menopause, with 40% to 56% of women in this stage reporting sleep-related issues.¹⁰ The underlying pathological mechanisms involve:
- Estrogen receptor remodeling Estrogen withdrawal down-regulates transcription of the GAT-1 GABA transporter gene in the brain’s preoptic area, reducing extracellular GABA concentration and elevating nighttime awakening risk.¹¹
- Vasomotor symptoms Approximately 85% of menopausal women experience hot flashes. During episodes, rapid minor fluctuations in core body temperature disrupt sleep architecture.¹²
4. The mood-sleep vicious cycle: Anxiety, depression and sleep disorders
People with anxiety or depression frequently experience severe sleep disturbances, while chronic sleep disorders conversely increase susceptibility to mood disorders. Nightshift workers show a 40% higher likelihood of developing depression compared to daytime workers.¹³ Core interacting mechanisms include:
- Cortisol rhythm disruption Depression flattens hormonal rhythm amplitudes (e.g. melatonin and cortisol), impairing circadian gene expression patterns in the brain and perpetuating sleep disturbances.¹³
- Serotonin dysregulation As serotonin (5-HT) neurotransmission is circadian-controlled, chronic sleep loss disrupts its function, exacerbating or triggering depressive/anxious states.¹⁴
Nighttime restfulness, daytime energy, and radiant skin
By optimizing circadian rhythms, urolithin A promotes deeper nighttime sleep while boosting daytime NAD+ levels for sustained energy. Simultaneously, it enhances skin vitality through improved collagen synthesis – creating a visible link between quality rest and radiant complexion that bridges internal wellness with outward vitality.
1. Dual-axis circadian regulation: Gut-to-body clock synchronization
- Gut barrier rhythm restoration Circadian rhythms play a pivotal role in maintaining intestinal homeostasis and immune function. Urolithin A effectively regulates core clock gene expression in gut epithelial cells, reversing inflammation-induced desynchronization between clock genes and tight junction proteins. In animal models, urolithin A restored normal expression rhythms of both tight junction proteins (Clnd1, Clnd4) and clock genes (BMAL1, PER2) in colonic tissue, while simultaneously modulating the central clock in the hypothalamic suprachiasmatic nucleus (SCN).¹⁵
- Senescent cell rhythm reprogramming The circadian clock precisely coordinates physiological processes, but aging impairs its temporal precision and robustness, manifesting as prolonged rhythms and dampened oscillation amplitudes. In senescent TIG-3 cell models, urolithin A dose-dependently enhanced BMAL1 promoter-driven luciferase rhythm amplitude by up to fourfold, demonstrating profound functional restoration of circadian regulation in aged cells.¹⁶
2. Boosting endogenous NAD+ synthesis for cellular energy
Studies show urolithin A significantly elevates cellular NAD+ levels, boosting energy metabolism and daytime vitality. In mice studies, urolithin A supplementation increased NAD+ levels by 50% – equivalent to 5x the efficacy of nicotinamide riboside (NR).¹⁷
Notably, urolithin A enhances NAD+ through endogenous synthesis via SIRT1-NAMPT pathway activation, distinct from exogenous precursor supplementation. This metabolic reprogramming approach provides theoretical support for long-term intervention safety.¹⁷
3. Combating sleep-deprivation fatigue and enhancing daytime performance
In sleep-deprived mouse models, urolithin A demonstrated superior anti-fatigue effects compared to caffeine: a 35% improvement in grip strength (vs. 12% with caffeine), a 100% prolongation of fatigue resistance in Rota-rod tests, and a simultaneous improvement in gut microbiota imbalance.¹⁸
4. Circadian-driven skin rejuvenation: The science of ‘beauty sleep’
Sleep serves as nature’s skin rejuvenator, with effects mediated through circadian rhythms, hormonal balance, and barrier function. Chronic sleep deprivation accelerates skin aging across genetic, metabolic, and structural levels – making quality sleep more cost-effective than premium skincare.
In terms of the mechanism, during nighttime repair cycles, urolithin A enhances BMAL1 rhythm amplitude in dermal fibroblasts, upregulating collagen synthesis genes (e.g. COL1A1) to promote barrier repair and elastic fiber regeneration.

Evidence-based research on sleep and anti-aging
In 2025, Bonerge started a clinical trial (Registration #NCT06990256) investigating urolithin A’s effects on sleep quality, aiming to address sleep disorders from an aging biology perspective.
This randomized, quadruple-blind (participants, healthcare providers, researchers, and assessors), placebo-controlled study will enroll 80 participants aged 45-70, divided into four groups: urolithin A group, fisetin group, urolithin A+ fisetin group and placebo control. The 12-week intervention marks the first systematic evaluation of urolithin A’s potential in resynchronizing circadian rhythms and mitigating age-related sleep disturbances.
Key study design features
Inclusion criteria Targets subclinical populations with impaired sleep quality (PSQI score >5) but without pathological diagnosis, excluding caffeine/alcohol-dependent individuals to ensure intervention purity.
Multidimensional assessment:
- Subjective measures Sleep quality scores, chronotype questionnaire (morning/evening preference), daytime dysfunction scales.
- Objective measures Continuous actigraphy monitoring, polysomnography (PSG) analysis.
- Biomarkers NAD+ levels, DNA methylation age, inflammatory markers (plasma IL-6, TNF-α), cortisol rhythm, circadian proteins (BMAL1, PER2), insulin resistance (HOMA-IR), and immunoglobulin levels.
“We’re not just tracking sleep duration or fragmentation – we aim to uncover the causal relationship between sleep disorders and aging through epigenetic clocks and metabolic homeostasis markers,” says Professor Chen, the lead investigator of the study.
“The combination of urolithin A (circadian regulator) and fisetin (senolytic) explores synergistic effects, potentially offering novel solutions for complex conditions like menopausal sleep disorders.”
Scientific commitment: From ‘ingredient innovation’ to ‘health ecosystem’
As a leading producer of high-quality ingredients, Bonerge’s R&D strategy follows a comprehensive ‘ingredient science, mechanistic research, clinical translation’ pipeline. Bonerge is now actively seeking global clinical partners from academia and industry to jointly explore urolithin A’s potential in sleep health, cellular aging intervention, and beyond.
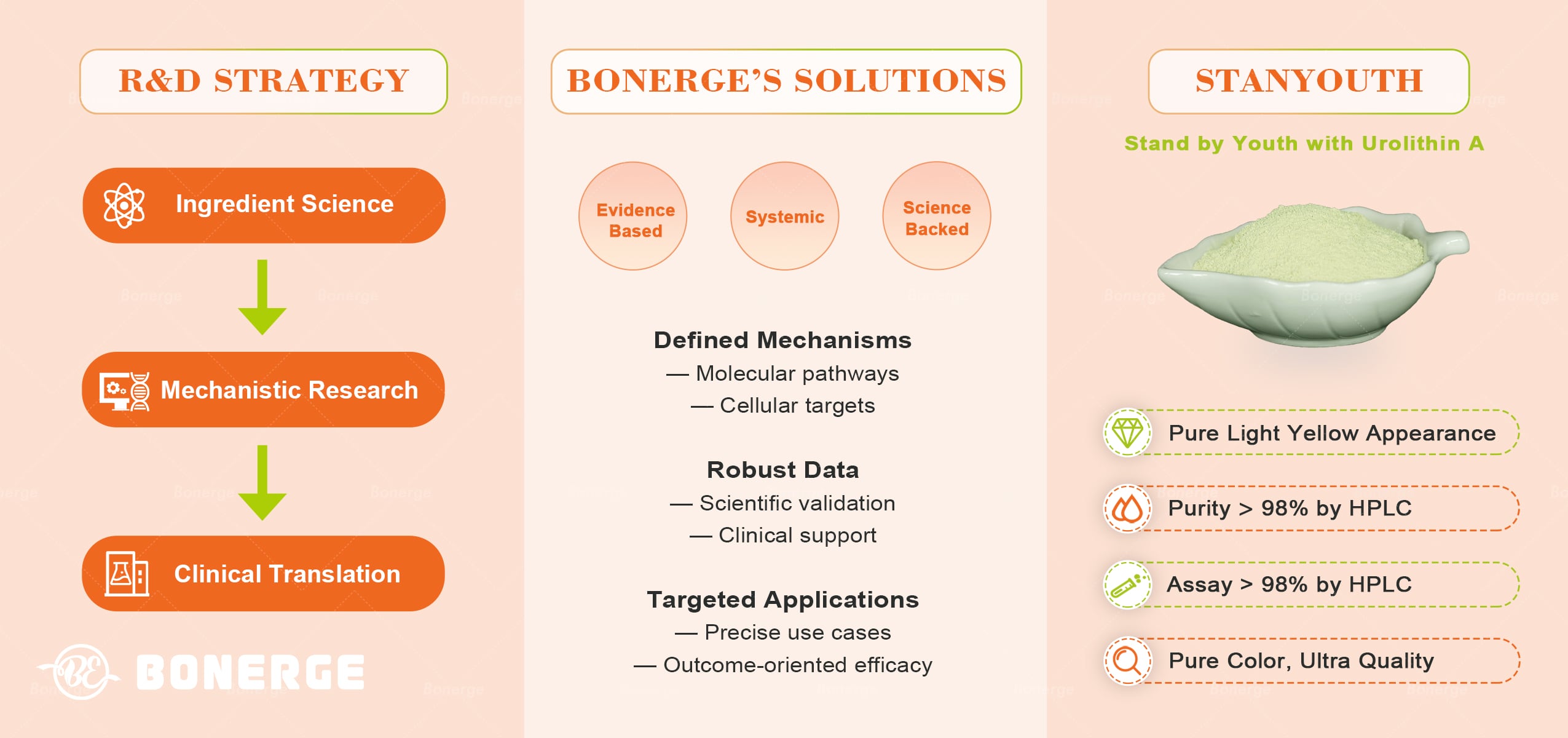
As the anti-aging narrative shifts from consumerism to scientific rigor, Bonerge leverage urolithin A not merely as a standalone ingredient, but as a catalyst for the industry’s return to evidence-based solutions.
From molecular mechanisms to clinical endpoints, the future of anti-aging must be built on clearly defined mechanisms, robust data, and precisely targeted applications – delivering systemic, science-backed solutions.
References
- San, L.; et al. The Night and Day Challenge of Sleep Disorders and Insomnia: A Narrative Review. Actas Esp Psiquiatr, 2024 Feb 5;52(1):45-56.
- Bhagavan, SM.; et al. Sleep Fragmentation and Atherosclerosis: is There a Relationship? Mo Med. 2021 May-Jun;118(3):272-276.
- Junxiu, W.; et al. China Sleep Research Report[M]. Social Sciences Academic Press, 2025.
- Feeney, S.P.; et al. Sleep loss is a metabolic disorder. Sci Signal. 2025 Apr 8;18(881):eadp9358. doi: 10.1126/scisignal.adp9358. Epub 2025 Apr 8.
- Carreras, A.; et al. Chronic sleep fragmentation induces endothelial dysfunction and structural vascular changes in mice. Sleep. 2014 Nov 1;37(11):1817-24.
- Bonmati-Carrion, M.A.; et al. Protecting the melatonin rhythm through circadian healthy light exposure. Int J Mol Sci. 2014 Dec 17;15(12):23448-500.
- Boivin, D.B.; et al. Disturbance of the Circadian System in Shift Work and Its Health Impact. J Biol Rhythms. 2022 Feb;37(1):3-28.
- Mosure, S.A.; et al. Targeting Nuclear Receptors for TH17-Mediated Inflammation: REV-ERBerations of Circadian Rhythm and Metabolism. Immunometabolism. 2022 Apr 18;4(2):e220006.
- Yang, Z.; et al. Liver-gut axis signaling regulates circadian energy metabolism in shift workers. FASEB J. 2024 Nov 30;38(22):e70203.
- Kravitz, H.M.; et al. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003 Jan-Feb;10(1):19-28.
- Herbison, A. E.; (1997). Estrogen regulation of GABA transmission in rat preoptic area. Brain research bulletin, 44(4), 321–326.
- Santoro, N.; et al. Menopausal Symptoms and Their Management. Endocrinol Metab Clin North Am. 2015 Sep;44(3):497-515.
- Walker, WH 2nd.; et al. Circadian rhythm disruption and mental health. Transl Psychiatry. 2020 Jan 23;10(1):28.
- Pourhamzeh, M.; et al. The Roles of Serotonin in Neuropsychiatric Disorders. Cell Mol Neurobiol. 2022 Aug;42(6):1671-1692.
- Du, Y.; et al. Effect of Urolithin A on the Improvement of Circadian Rhythm Dysregulation in Intestinal Barrier Induced by Inflammation. Nutrients. 2024 Jul 13;16(14):2263.
- Kuatov, R.; et al. Urolithin A Modulates PER2 Degradation via SIRT1 and Enhances the Amplitude of Circadian Clocks in Human Senescent Cells. Nutrients. 2024 Dec 25;17(1):20.
- Ghosh, N.; et al. Urolithin A augments angiogenic pathways in skeletal muscle by bolstering NAD+ and SIRT1. Sci Rep. 2020 Nov 19;10(1):20184.
- Zhu, H.; et al. Urolithin A Ameliorates Athletic Ability and Intestinal Microbiota in Sleep Deprivation from the Perspective of the Gut-Muscle Axi. Mol Nutr Food Res. 2024 Apr;68(7):e2300599.





