The women’s health industry is growing, driven by rising awareness of gender-specific health needs and the demand for personalized, science-backed solutions.
In 2024 the global women’s health market size was estimated at US $49.33 billion, and is expected to grow at a CAGR of 5.1% from 2025 to 2030.¹
Among the emerging stars in this sector, S-equol – a gut microbiota-derived metabolite of soy isoflavones – has garnered significant attention for its unique ability to address critical health challenges such as menopause management, bone preservation, cardiovascular wellness, and skin health protection.
With its selective estrogen receptor modulation, clinically validated efficacy, and robust safety profile, S-equol is poised to redefine standards in women’s health. This article explores its scientific foundations, multifaceted benefits, and market potential, emphasizing its role as a transformative agent in modern healthcare.
The development history of S-equol
The story of S-equol began in 1932 when German scientists first isolated equol from the urine of pregnant mares.² However, its relevance to human health remained unclear until 1984, when Japanese researchers identified it as the final metabolite of soy isoflavones in human urine, establishing its critical role in nutritional metabolism.²
Modern molecular biology research has further revealed that naturally occurring equol has two enantiomers, (S)-equol and (R)-Equol. And a pivotal breakthrough occurred in 2005, when Dr. Kenneth Setchell’s team discovered that the natural isomer produced by the metabolism of gut microbiota is S-equol.³
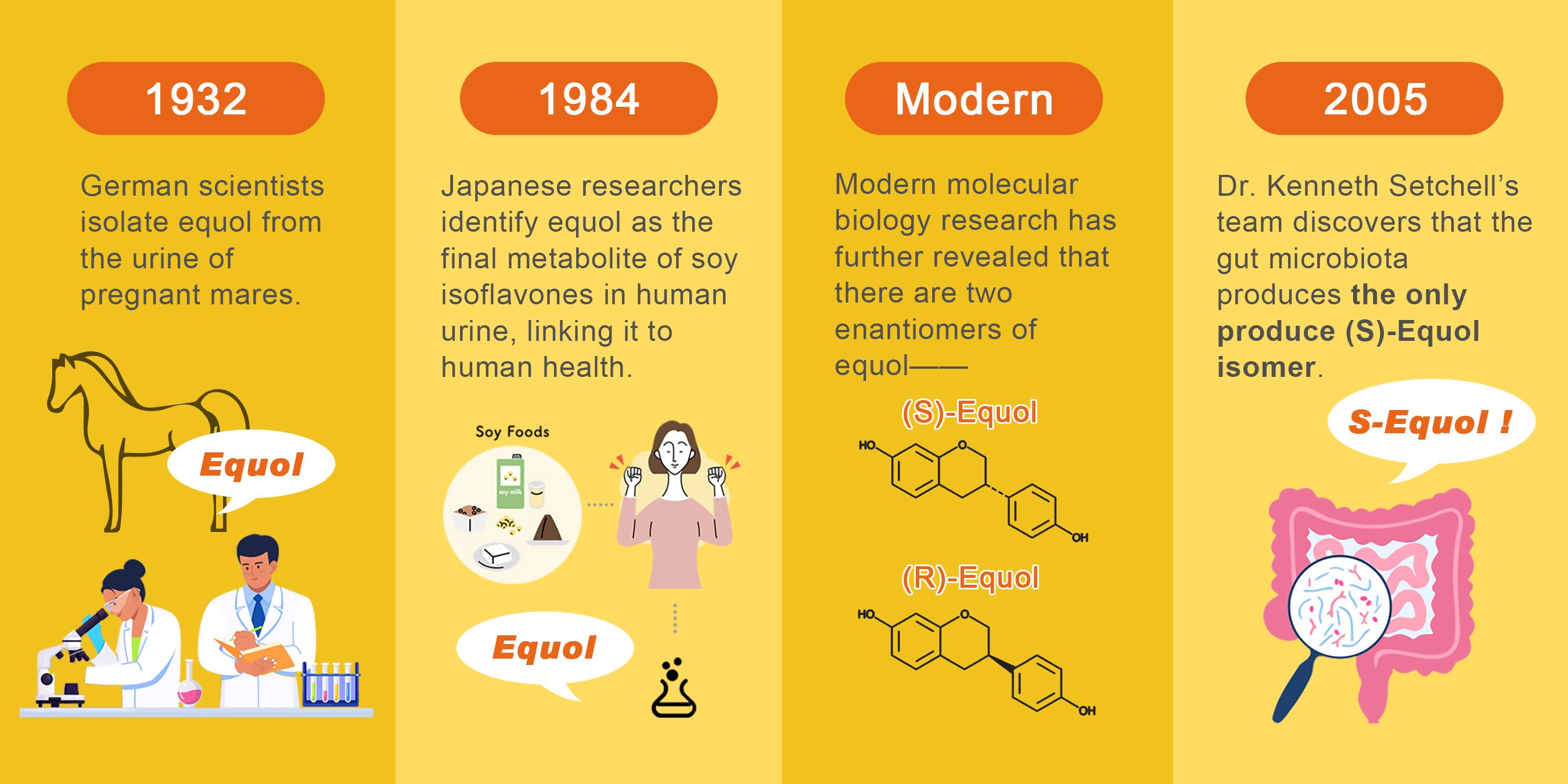
Structural analysis via X-ray crystallography revealed that S-equol’s hydroxyl groups align spatially with those of 17β-estradiol, the most potent endogenous estrogen. This structural similarity enables S-equol to bind selectively to estrogen receptor beta (ERβ) while avoiding activation of ERα, a key feature that underpins its therapeutic specificity and safety.³
Biological activities: Comprehensive health benefits
- Menopause symptom relief
Menopause, characterized by estrogen decline, affects over 1.3 billion women globally, with symptoms including hot flashes, night sweats, mood swings, and sleep disturbances.
In nutraceutical markets, menopausal supplements are primarily formulated with soy isoflavones – phytoestrogens that exert ERβ-selective agonism without activating ERα – which can circumvent estrogenic side effects associated with ERα-mediated proliferative pathways.
As the terminal metabolite of soy isoflavones, S-equol retains this ERβ selectivity while exhibiting enhanced bioavailability and structural stability. The parallel artificial membrane permeability assays (PAMPA) further demonstrate S-equol’s superior gastrointestinal absorption and blood-brain barrier (BBB) penetration compared to precursor isoflavones like daidzein or genistein, likely due to its reduced molecular polarity.⁴
So S-equol activates ERβ-mediated transcriptional pathways to exert estrogenic effects in low-estrogen environments, effectively alleviating menopausal symptoms.
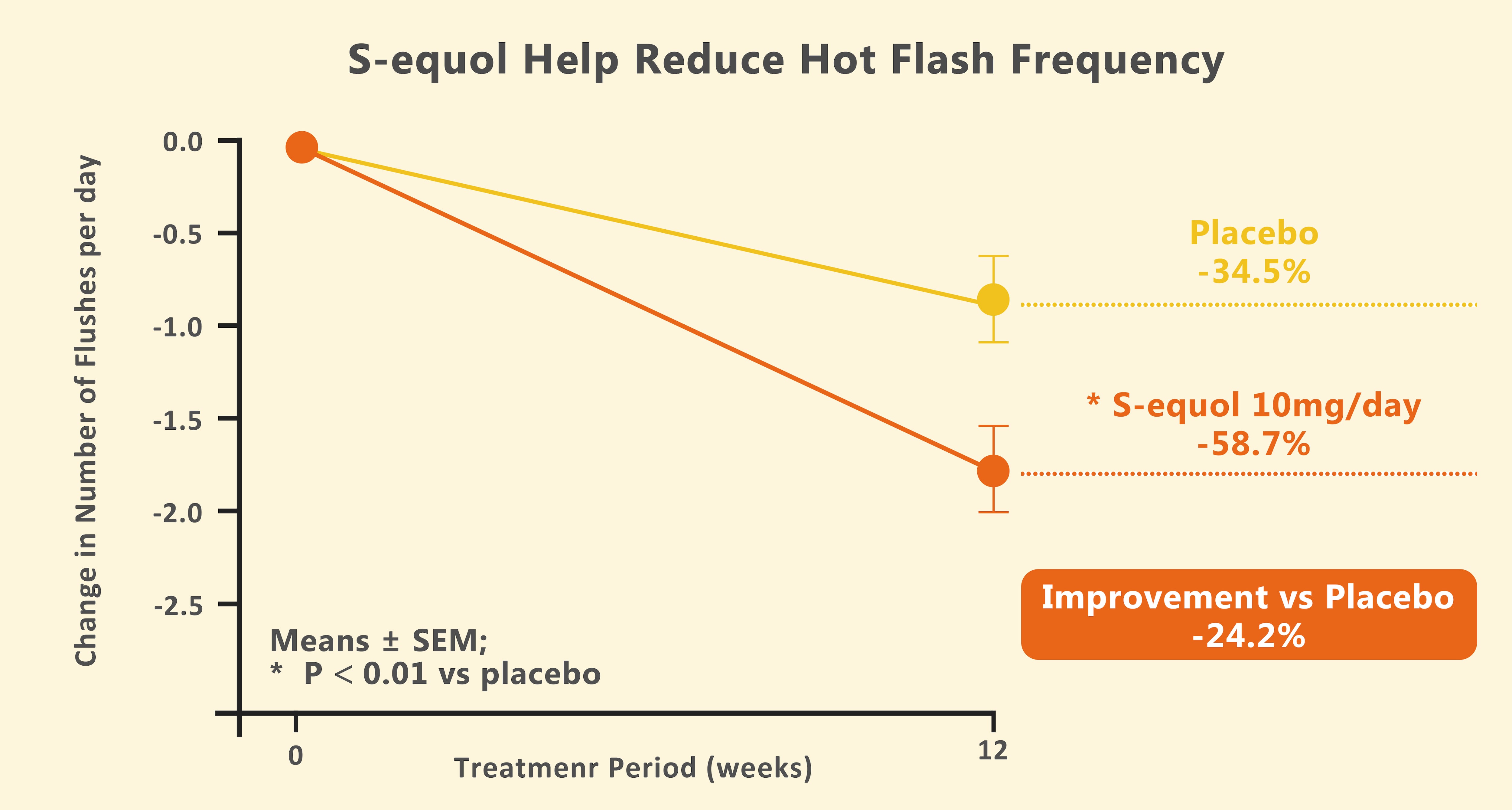
A 12-week randomized controlled trial (RCT) involving 114 postmenopausal women demonstrated that 10mg/day S-equol reduced hot flash frequency by 58.7% (p<0.01 vs. 34.5% with placebo), alongside significant improvements in cervicobrachial muscle stiffness.⁵
In another 12-week, randomized, double-blind, placebo-controlled trial of 134 Japanese women aged 40–59, daily intake of 10mg S-equol (three times/day) significantly reduced depression scores and improved mood state scale measures – including tension-anxiety, depression-despondency, and fatigue – compared to placebo. Vitality scores also increased significantly, suggesting S-equol effectively supports menopausal emotional health.⁶
- Bone health preservation
Postmenopausal bone loss accelerates at 2-3% annually, increasing osteoporosis risk. S-equol counteracts this by promoting osteoblast differentiation via the PI3K/Akt pathway and suppressing RANKL-mediated osteoclastogenesis.
In a one-year, double-blind, randomized, placebo-controlled trial involving 93 S-equol non-producing Japanese postmenopausal women, daily supplementation with 10mg S-equol significantly reduced urinary deoxypyridinoline (a bone resorption marker) by 23.94% after 12 months, compared to a -2.87% change in the placebo group.
The findings demonstrate that 10mg daily S-equol can not only effectively suppresses bone resorption, but can also prevent the decline in whole-body bone mineral density.⁷ These results indicate that S-equol supplementation can support bone health without adverse effects.
- Cardiovascular health support
A prospective observational study of 74 women receiving 10mg daily equol supplementation for one year showed improvements in cardiovascular parameters.⁸ Arterial stiffness decreased overall, with significant reductions in women at moderate (3.2%) and high (12.65%) arteriosclerosis risk.
Hypertriglyceridemia patients showed a 45.53% decrease, while the 15 women with elevated baseline parathyroid hormone levels experienced a 50% reduction. Multiple linear regression analysis confirmed these associations, demonstrating that one-year equol supplementation is well-tolerated and improves cardiovascular markers, particularly in high-risk groups.⁸
- Skin health improvement
Menopause can accelerate skin aging, with a 30% loss of dermal collagen within five years. Clinical trials show that daily supplementation with 30mg S-equol for 12-weeks reduces wrinkle depth by 18% and area by 22%, outperforming placebo. This effect is attributed to S-equol’s ability to inhibit collagen-degrading enzymes like MMP-1 while enhancing skin elasticity.⁹
Safety assessment: Rigorous evaluation for clinical confidence
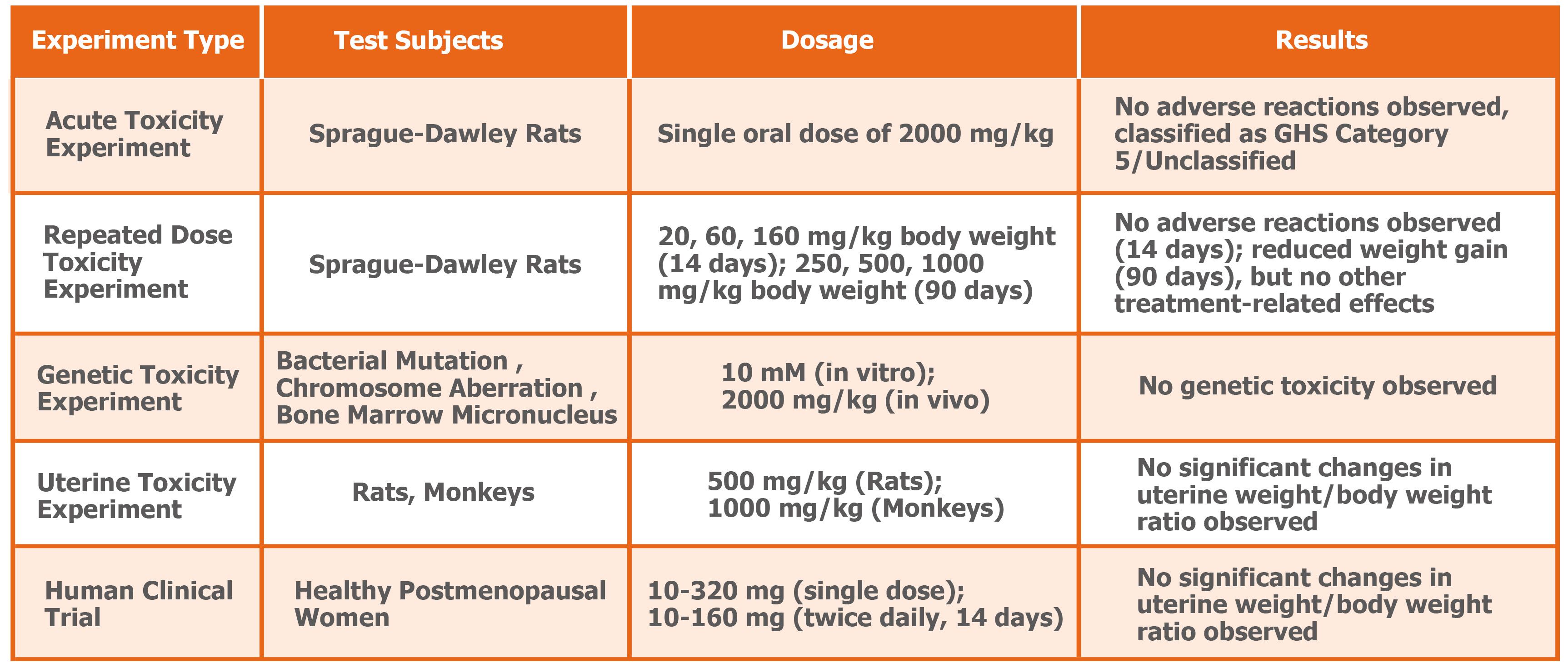
S-equol’s safety is well-established through preclinical and clinical studies. In acute oral toxicity studies, rats tolerated doses exceeding 2000mg/kg. A 14-day repeated dose study determined the NOAEL (no observed adverse effect level) at 160mg/kg/day – 242 times the human daily intake.
A 90-day study also found no treatment-related adverse effects, supporting long-term safety.¹⁰ Mutagenicity tests (Ames and micronucleus assays) were negative, indicating no genotoxic risk. Reproductive toxicity studies further confirmed no impact on ovarian reserve or fertility.¹¹⁻¹²
Human studies have further supported S-equol’s safety. In two randomized, double-blind, placebo-controlled trials with healthy volunteers – a single-dose study (10-320mg, n=61) and a 14-day multiple-dose study (10-160mg twice daily, n=40) – all participants tolerated S-equol well with no major drug-related adverse events.¹³ These results demonstrate S-equol’s excellent safety profile at recommended doses.
Market trends: Growth, diversification, and personalization
The global menopause market size was estimated at USD 16.93 billion in 2023 and is expected to grow at a compound annual growth rate (CAGR) of 5.37% from 2024 to 2030. Increasing prevalence of postmenopausal symptoms owing to a shift towards a sedentary lifestyle is expected to drive market growth.¹⁴
The S-equol market is expanding rapidly, fueled by growing demand for natural, evidence-based supplements. Innovations include synergistic blends with vitamin D3, omega-3 fatty acids, and probiotics, reflecting a shift toward holistic, personalized health solutions.
A key driver is the ‘equol producer’ paradigm: only 20–35% of adults naturally produce S-equol due to gut microbiota composition, particularly the presence of Slackia isoflavoniconvertens.² To address this, companies are pursuing dual strategies:
Direct supplementation Providing ready-to-use S-equol for non-producers.
Microbiome modulation Developing probiotic formulations to enhance endogenous S-equol synthesis.
A new frontier in women’s health
S-equol represents a paradigm shift in addressing women’s health challenges, merging cutting-edge science with natural biochemistry. Its ability to selectively modulate Erβ – while avoiding ERα-related risks – positions it as a safer, more effective alternative to some traditional therapies.
With innovators like Bonerge Lifescience harnessing patented technology to achieve 99% pure S-equol production, the horizons for this compound stretch far beyond its current uses. The next frontier? Rigorous long-term clinical studies – and pioneering combinations with prebiotics or cutting-edge ingredients like fisetin, urolithin A, and pyrroloquinoline quinone (PQQ) to unlock synergistic benefits.

In an era where women increasingly seek natural, safe, and personalized health solutions, S-equol stands at the forefront of innovation, offering a scientifically validated pathway to healthier aging. By bridging dietary wisdom with molecular precision, it exemplifies the transformative power of translational science in modern healthcare.
References
- Grand View research. Women’s Health Market Size, Share & Trends Analysis Report By Application (Contraceptives, Menopause), By Drug Class (Hormonal Therapies, Pain And Symptom Management), By Age (50 Years And Above), By Region, And Segment Forecasts, 2025 – 2030.
- Setchell, K. D.; et al. Equol: history, chemistry, and formation. The Journal of nutrition, 2010, 140(7): 1355s- 62s.
- Setchell, K. D.; et al. S-equol, a potent ligand for estrogen receptor beta, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. American Journal of Clinical Nutrition, 2005, 81(5): 1072-1079.
- Sekikawa, A.; et al. Potential Protective Mechanisms of S-equol, a Metabolite of Soy Isoflavone by the Gut Microbiome, on Cognitive Decline and Dementia. International journal of molecular sciences, 2022, 23(19).
- Aso, T.; et al. A natural S-equol supplement alleviates hot flushes and other menopausal symptoms in equol nonproducing postmenopausal Japanese women. J Womens Health 2012; 21:92-100.
- Ishiwata, N.; et al. New equol supplement for relieving menopausal symptoms: randomized, placebo-controlled trial of Japanese women. Menopause, 2009, 16(1): 141-148.
- Tousen, Y.; et al. Natural S-equol decreases bone resorption in postmenopausal, non-equol-producing Japanese women: a pilot randomized, placebo-controlled trial. Menopause, 2011, 18(5): 563-574.
- Yoshikata R, et al. Effects of equol supplement on bone and cardiovascular parameters in middle-aged japanese women: A prospective observational study. Journal of Alternative and Complementary Medicine, 2018, 24(7): 701-708.
- Oyama, A.; et al. The effects of natural S-equol supplementation on skin aging in postmenopausal women: a pilot randomized placebo-controlled trial. Menopause, 2012, 19(2): 202-210.
- AnandaKumar, S. R.; et al. Safety assessment of (S)-Equol: Subchronic toxicity study in Sprague Dawley Rats. Toxicology Reports, 2024, 13: 101823.
- Schwen, R.; et al. Genotoxicity assessment of S-equol in bacterial mutation, chromosomal aberration, and rodent bone marrow micronucleus tests. Food and Chemical Toxicology, 2010, 48(12): 3481-3485.
- Schwen, R.J.; et al. Toxicokinetics and lack of uterotropic effect of orally administered S-equol. Food Chem Toxicol. 2012. 50(5): 1741–1748.
- Jackson, R.L.; et al. Single-dose and steady-state pharmacokinetic studies of S-equol, a potent nonhormonal, estrogen receptor A-agonist being developed for the treatment of menopausal symptoms. Menopause, 2011, 18(2): 185-193.
- Grand View research. Menopause Market Size, Share & Trends Analysis Report By Treatment (Dietary Supplements, OTC Pharma Products), By Region (North America, Europe, Latin America), And Segment Forecasts, 2024 – 2030.





