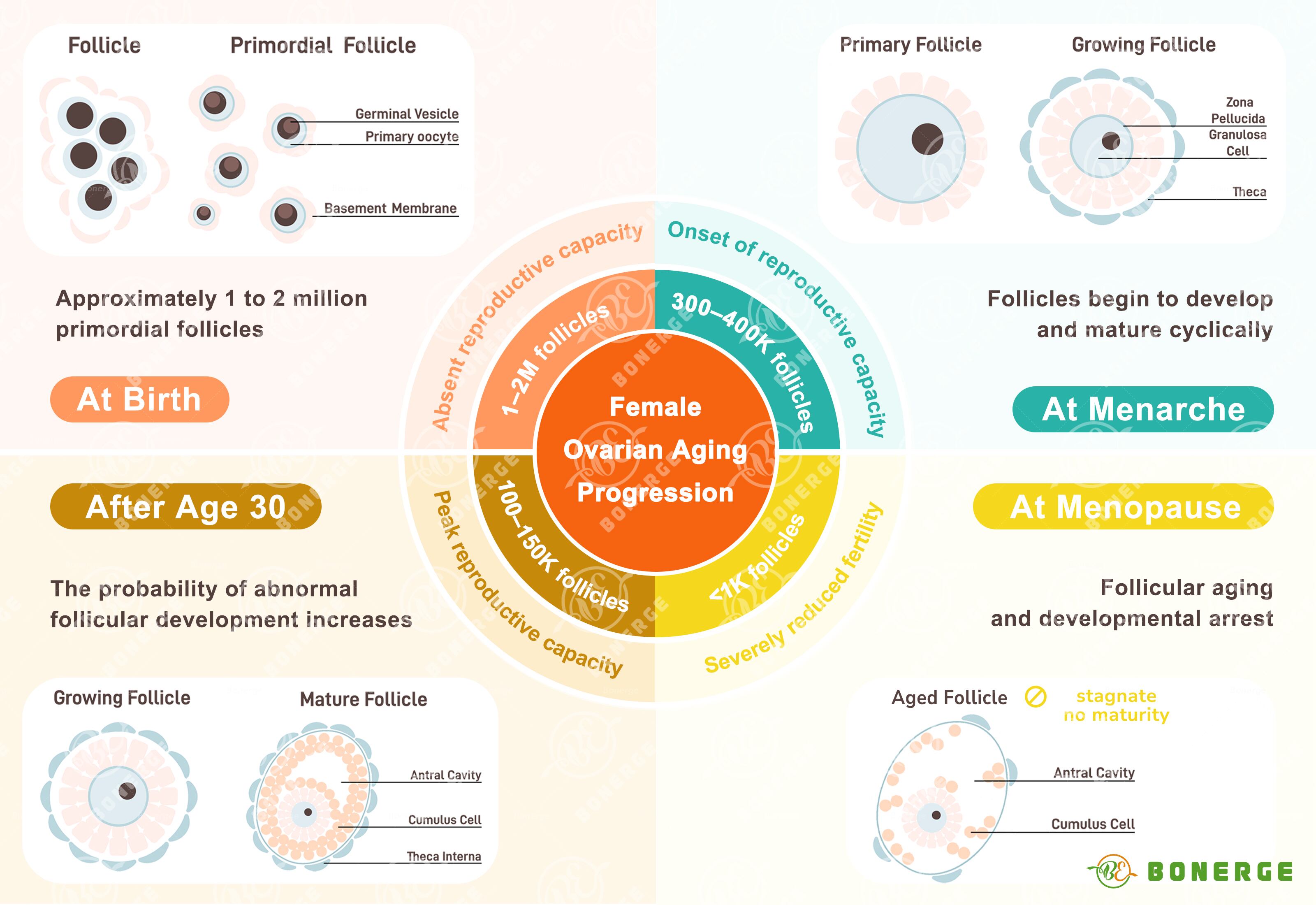
We readily notice wrinkles or grey hair as signs of aging, yet few realize that the ovaries are among the fastest-aging organs in the female body.¹
Ovarian function begins its decline around age 30, accelerating significantly after 35 – a stage medically termed ‘advanced maternal age’. However, some women delay childbearing until after 35, prioritizing careers and education first. By the time fertility issues surface, the optimal window for intervention may have narrowed considerably. Understanding the science behind fertility decline is therefore essential for proactive reproductive health management.
Why ovaries age faster: Understanding female fertility decline
The decline in ovarian function is the hallmark of female reproductive aging, characterized primarily by:²
Reduction in oocyte (egg) reserve Normally, women are born with one to two million primordial follicles. Through continuous atresia (natural degeneration), approximately 90% are lost by age 30. This number further dwindles to less than 1,000 by menopause, leading to an accelerated depletion of the ovarian reserve.
Decline in oocyte quality Oocyte quality progressively deteriorates after age 30. The frequency of meiotic errors (aneuploidy) increases, directly raising the risk of embryonic chromosomal abnormalities. This manifests clinically as higher rates of miscarriage, pregnancy loss, or chromosomal disorders (e.g. Down syndrome).
Uterine structural and functional changes Uterine tissues undergo fibrosis and expansion, reducing hormonal responsiveness. Declining levels of estrogen and progesterone elevate risks of osteoporosis and cardiovascular disease, and trigger menopausal symptoms like hot flashes, insomnia, and emotional lability.
Cellular senescence: How ‘zombie cells’ accelerate ovarian aging
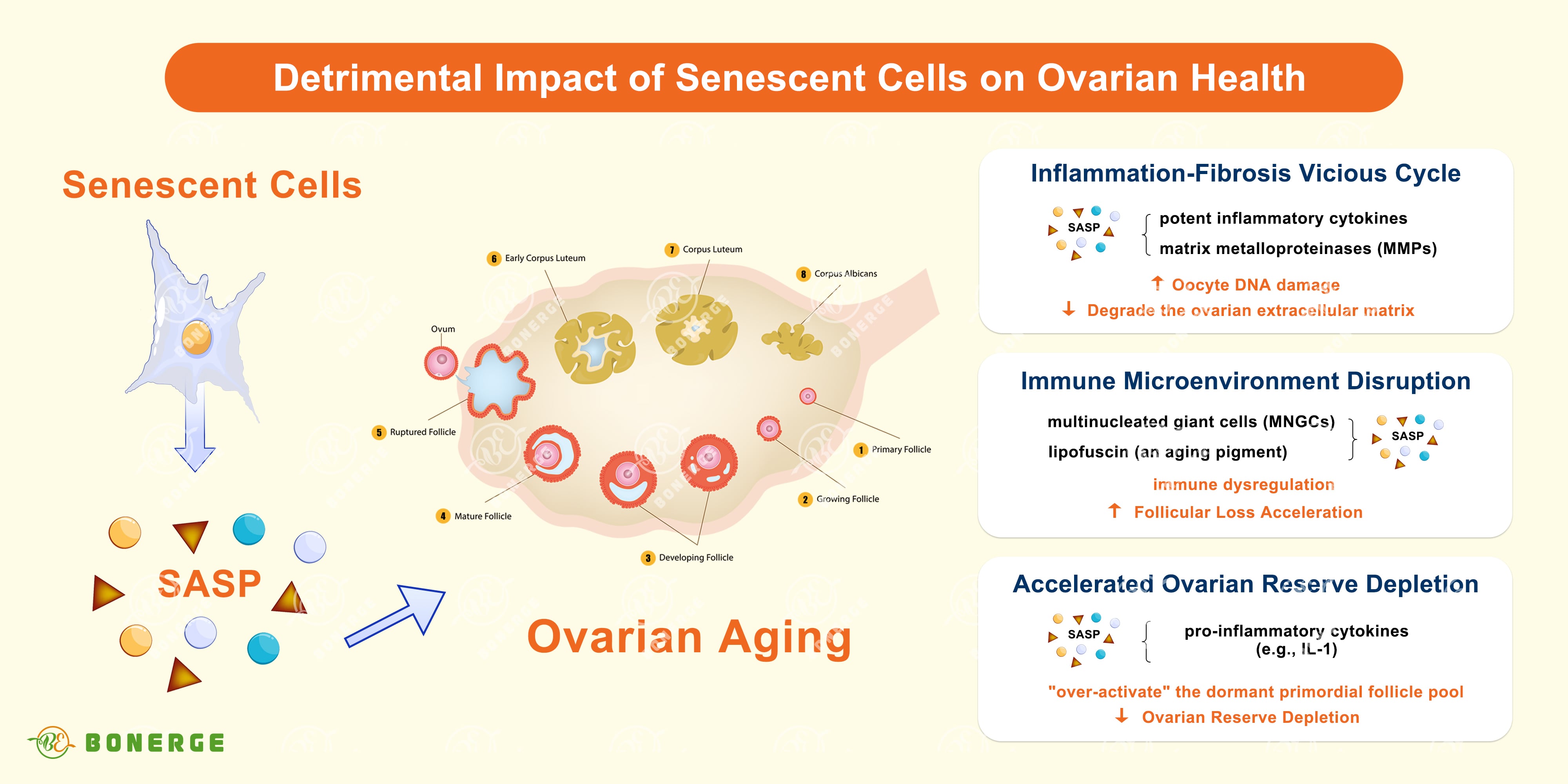
Ovarian aging is a process tightly linked to the abnormal accumulation of senescent cells (also known as ‘zombie cells’). Far from being inert, these ‘zombie cells’ secrete a toxic cocktail of signals known as the Senescence-Associated Secretory Phenotype (SASP), which actively drives ovarian functional decline.³
Cellular senescence is a state where cells permanently cease division but remain metabolically active, persistently secreting inflammatory and tissue-damaging factors (SASP), thereby accelerating ovarian decay. SASP not only damages adjacent healthy cells but can also disrupt distant tissues.
Critically, ovarian senescent cell accumulation exhibits early onset and rapid progression:³
Animal model evidence In mice, key senescence markers (p16, p21, lipofuscin) increase significantly in ovaries between 3-12 months (equivalent to human youth to middle age), whereas pronounced aging signs in organs like liver and kidney typically appear much later, at 18-20 months (equivalent to human old age).
Human tissue evidence Ovarian stromal tissue from women over 37 years shows elevated p21 expression and activation of fibrosis-related genes. External stressors such as chemotherapy or obesity can markedly exacerbate these senescence-related changes.
How do senescent cells affect ovarian function?
Inflammation-fibrosis vicious cycle³ Senescent cells secrete potent inflammatory cytokines (e.g. IL-6, TNF-α) and matrix metalloproteinases (MMPs). These factors directly damage oocyte DNA and recruit pro-inflammatory M1 macrophages, creating a self-perpetuating ‘inflammation-senescence’ feedback loop. MMPs degrade the ovarian extracellular matrix, leading to collagen misalignment, increased tissue stiffness, and impaired ovulation.
Immune microenvironment disruption³ Advanced single-cell sequencing reveals that a significant portion of ovarian senescence signals originate from immune cells. With advancing age, structures like multinucleated giant cells (MNGCs, likely formed by fused macrophages) co-localize with lipofuscin (an aging pigment), indicating senescent immune phenotypes. This immune dysregulation actively accelerates follicular loss.
Accelerated ovarian reserve depletion³ Pro-inflammatory factors secreted by senescent cells can ‘over-activate’ the dormant primordial follicle pool, pushing them into growth phases prematurely and triggering irreversible atresia instead of ovulation. Conversely, studies on IL-1 knockout mice (lacking key inflammatory factors) show extended ovarian lifespan and increased follicle reserves, highlighting the detrimental role of inflammation.
Senescent cells: Also a key driver of male fertility decline
While women face a pronounced early decline in ovarian function, male fertility notably declines after age 40. Although men have a longer potential reproductive window, advancing age reduces sperm count and quality and increases the risk of erectile dysfunction. Cellular senescence is increasingly recognized as a core driver of male fertility decline, impacting both spermatogenesis and hormone regulation.⁴
Direct impacts of cellular senescence on the male reproductive system include:⁴
Decline in spermatogonial stem cell (SSC) proliferation
In aged mouse testes, the population of PLZF+ spermatogonia (a key stem cell marker) significantly decreases, accompanied by a sharp drop in Ki67+ (actively proliferating) spermatogonia. This fundamentally impairs the testis’s sperm production capacity.
Testicular endothelial cell (EC) senescence and support function impairment
Senescence markers (like SA-β-gal+) surge dramatically in testicular endothelial cells of aged mice. This is associated with upregulated p21 expression and reduced EC proliferation. Critically, senescent ECs decrease secretion of crucial growth factors like GDNF and FGF (vital for SSC maintenance and function) and may instead secrete pro-inflammatory factors, creating a microenvironment inhibitory to spermatogenesis.
Seminiferous tubule degeneration
Aged mouse testes frequently show ‘degenerate’ seminiferous tubules – either completely devoid of germ cells or exhibiting severe structural defects. The thinning and loss of the germ cell layer results directly from impaired SSC proliferation and disrupted spermatogenesis cascades.
Fisetin: The natural senolytic powerhouse for fertility health
Fisetin, a potent natural flavonoid abundantly found in fruits like strawberries and persimmons, demonstrates remarkable protective potential for both male and female fertility health. Its exceptional ability lies in selectively clearing damaging senescent cells (‘senolytic’ activity), thereby addressing a root cause of reproductive aging.
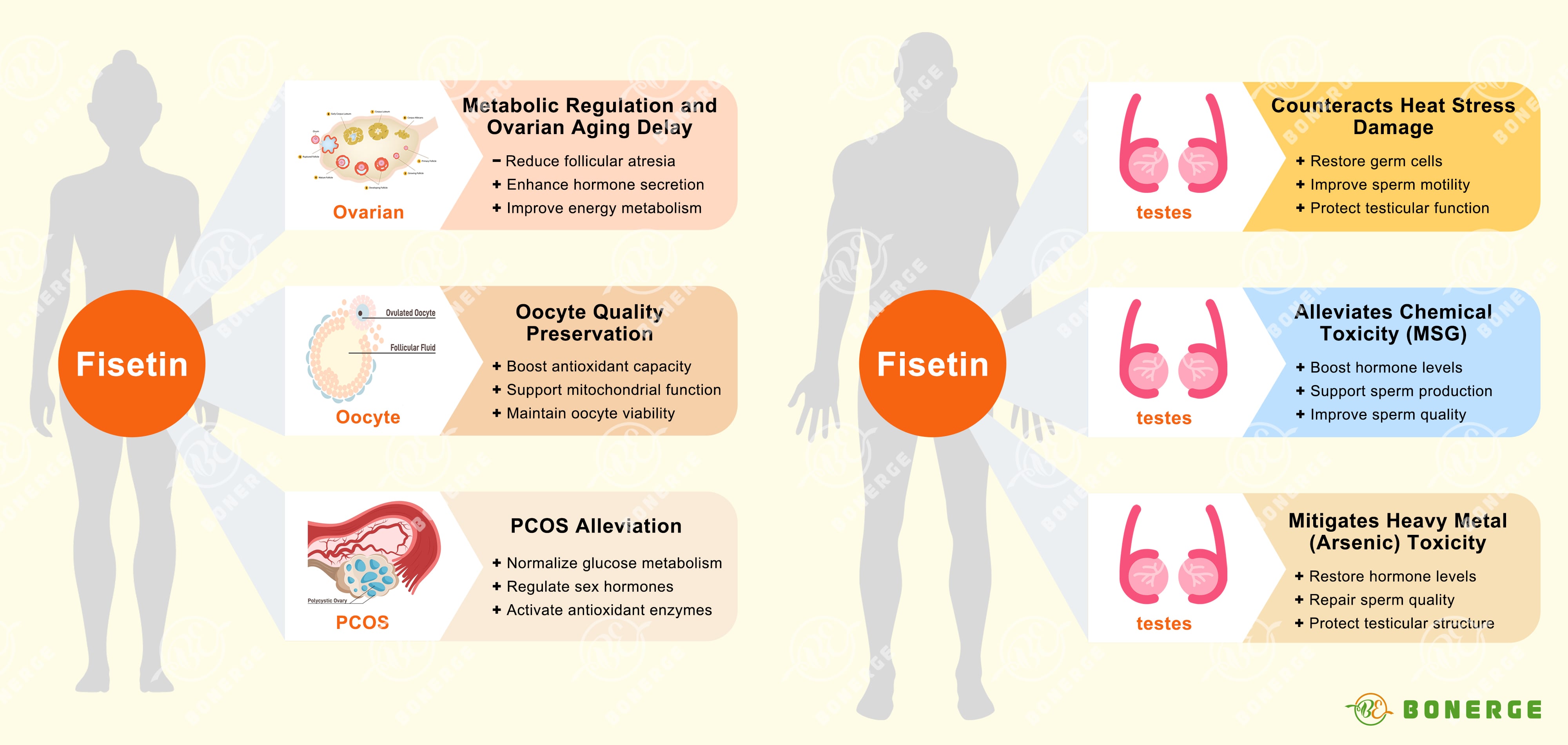
Fisetin safeguards female reproductive function:
Metabolic regulation and ovarian aging delay
In 2024, a pivotal study demonstrated that dietary fisetin supplementation significantly improved egg production and shell quality in aging hens.⁵ The mechanisms included reducing follicular atresia, promoting ovarian cell proliferation, elevating serum estrogen and progesterone levels, restoring antioxidant capacity, and optimizing energy metabolism.
Oocyte quality preservation
A 2023 study found that fisetin effectively delays post-ovulatory oocyte aging in mice. It achieves this by inhibiting harmful reactive oxygen species (ROS) accumulation through increasing glutathione (GSH) levels. Furthermore, fisetin upregulates Sirt1, which promotes the mitochondrial transcription factor Tfam and key mitochondrial genes (COX2, Atp8), ultimately enhancing the developmental potential of aged oocytes after fertilization.⁶
Polycystic ovary syndrome (PCOS) alleviation
Multiple animal studies show fisetin normalizes disrupted glucose/lipid profiles, improves the insulin resistance index (HOMA-IR), and restores balanced testosterone/estradiol levels in PCOS model mice.⁷⁻⁸ The mechanisms involve upregulating SIRT1 and AMPK pathways (central regulators of energy metabolism and inflammation) and restoring the activity of crucial antioxidant enzymes (CAT, SOD, GPx). Notably, fisetin demonstrated comparable effectiveness to the standard drug metformin in alleviating core PCOS symptoms.⁷⁻⁸
Fisetin protects male fertility health
Fisetin can cross the blood-testis barrier to exert protective effects on reproductive endocrine function and sperm quality:
Counteracts heat stress damage
A 2021 study demonstrated that fisetin (administered either preventatively or therapeutically) significantly improved spermatogenic dysfunction in mice caused by chronic scrotal hyperthermia.⁹ Benefits included restored testicular volume, increased numbers of spermatogonia, primary spermatocytes, round spermatids, and sertoli cells, alongside significant improvements in sperm count, motility, and key testicular biochemical markers.⁹
Alleviates chemical toxicity (MSG/monosodium glutamate)
In rats suffering with testicular toxicity induced by MSG, fisetin provided dual protection: centrally by modulating the hypothalamic-pituitary-gonadal (HPG) sex hormone axis, and peripherally by activating the SIRT1/pAMPK pathway and exerting potent antioxidant effects directly within testicular tissue. This combined action promoted normal sex hormone secretion, supported spermatogenesis and improved overall sperm quality.¹⁰
Mitigates heavy metal (arsenic) toxicity
Fisetin combats arsenic-induced testicular damage through multiple mechanisms: potent antioxidant activity, inhibition of lipid peroxidation, anti-apoptotic effects, and maintenance of sex hormone balance. This effectively reverses arsenic-induced declines in testosterone and gonadotropins, repairs sperm quality parameters, and ameliorates pathological damage to testicular structure.¹¹
The gold standard in senolytic fertility health support
BeFisetin®, a premium fisetin raw material developed by Bonerge Lifescience, is the first fisetin to achieve Self-Affirmed GRAS (Generally Recognized As Safe) status and initiate human clinical trials, establishing a new benchmark for quality and scientific validation in fertility and aging health.

BeFisetin has completed one human clinical trial that confirms its safety and preliminary efficacy for skin anti-aging. A randomized quadruple-blind controlled trial investigating its effects on sleep and immune health is currently ongoing (Registration #NCT06990256).
The third clinical trial directly targeting female ovarian health and premature ovarian insufficiency (POI) has been initiated and is in active protocol development. BeFisetin remains deeply committed to advancing clinical research to explore more possibilities for fisetin applications, particularly in reproductive health.
Guided by the uncompromising principle ‘Be the Golden Standard of Fisetin’, BeFisetin prioritizes consumer safety and establishes global benchmarks for fisetin raw material quality. Every stage – from stringent raw material sourcing and selection to state-of-the-art manufacturing and rigorous quality control – adheres to meticulous precision and international standards.
BeFisetin persistently invests in foundational and applied scientific research to fully leverage fisetin’s senolytic effect and health-promoting potential. The mission is to deliver innovative, science-backed solutions for the significant challenges to reproductive health, cellular vitality, and overall wellbeing posed by the global aging phenomenon.
Fisetin has demonstrated compelling efficacy in animal models for counteracting fertility decline by selectively eliminating senescent cells, quenching inflammation, and restoring metabolic balance. As pioneering clinical trials progress, Bonerge anticipates gaining deeper, evidence-based insights into fisetin’s significant potential to intervene in preserving fertility health and defying the biological clock.
References
- Yang Q, Chen W, et al. NADase CD38 is a key determinant of ovarian aging. Nat Aging. 2024 Jan;4(1):110-128.
- Cavalcante MB, et al. Ovarian aging in humans: potential strategies for extending reproductive lifespan. Geroscience. 2023 Aug;45(4):2121-2133.
- Hense JD, Isola JVV, et al. The role of cellular senescence in ovarian aging. NPJ Aging. 2024 Jul 20;10(1):35.
- Ozawa M, Mori H, et al. Age-related decline in spermatogenic activity accompanied with endothelial cell senescence in male mice. iScience. 2023 Nov 13;26(12):108456.
- Yang Z, Zhang J, et al. Flavonoid Fisetin Alleviates Ovarian Aging of Laying Chickens by Enhancing Antioxidant Capacity and Glucose Metabolic Homeostasis. Antioxidants. 2024, 13(12), 1432.
- Xing X, Liang Y, et al. Fisetin Delays Postovulatory Oocyte Aging by Regulating Oxidative Stress and Mitochondrial Function through Sirt1 Pathway. Molecules. 2023 Jul 20;28(14):5533.
- Mihanfar A, Nouri M, et al. Ameliorative effects of fisetin in letrozole-induced rat model of polycystic ovary syndrome. J Steroid Biochem Mol Biol. 2021 Oct;213:105954.
- Chahal SK, Kabra A. Fisetin ameliorates polycystic ovary syndrome in rats via a mechanistic modulation of AMP-activated protein kinase and SIRT1 molecular pathway. Naunyn Schmiedebergs Arch Pharmacol. 2024 Dec;397(12):10017-10029.
- Pirani M, Ghaffari Novin M, et al. Protective Effects of Fisetin in the Mice Induced by Long-Term Scrotal Hyperthermia. Reprod Sci. 2021 Nov;28(11):3123-3136.
- Rizk FH, Soliman NA, et al. Fisetin ameliorates oxidative glutamate testicular toxicity in rats via central and peripheral mechanisms involving SIRT1 activation. Redox Rep. 2022 Dec;27(1):177-185.
- Ijaz MU, Haider S, et al. Mechanistic insight into the protective effects of fisetin against arsenic-induced reproductive toxicity in male rats. Sci Rep. 2023 Feb 22;13(1):3080.





