Data published in a letter in JAMA indicated melatonin gummy products are often inaccurately labeled.
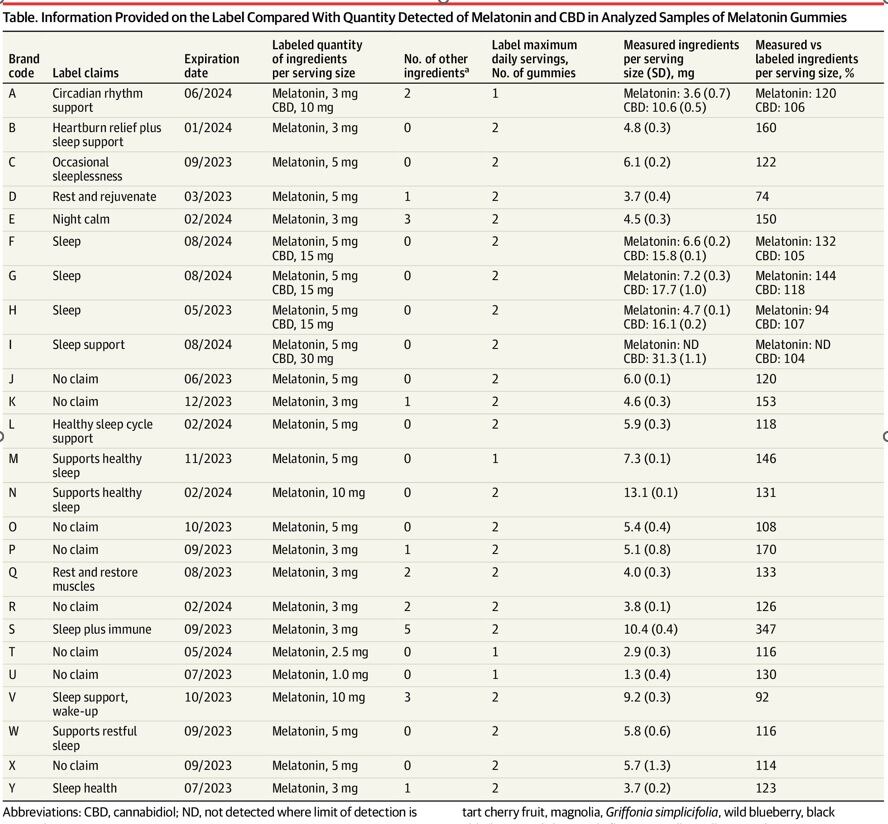
Of the 25 brands included in the gummy study, 22 were inaccurately labeled–with variations in melatonin spanning from 74% to 347% of the amount advertised on the label. This comes out to a range of melatonin that spanned 1.3 mg to 13.1 mg per serving. Only three out of 25 supplements matched the label within 10% of the amount advertised, and one supplement contained CBD instead of melatonin.
“Given these findings, clinicians should advise parents that pediatric use of melatonin gummies may result in ingestion of unpredictable quantities of melatonin and CBD,” the authors concluded.
The study has received widespread coverage, including articles in the New York Times, the Washington Post, and the Wall Street Journal.
Poison control calls
The authors said they wanted to take a closer look at melatonin gummies after last year’s report that poison centers received over a quarter million calls about pediatric ingestion.
According to the report, calls for pediatric melatonin ingestions to US Poison Control Centers increased 530% from 2012 to 2021 and were associated with 27,795 emergency department and clinic visits, 4,097 hospitalizations, 287 intensive care unit admissions, and 2 deaths.
Rick Kingston, PharmD, President, Regulatory and Scientific Affairs at SafetyCall International, said that when it comes to Poison Control calls, the totality of the experience– including the medical outcomes–must be considered.
“When you look at the actual outcomes associated with exposure calls to poison control centers, the safety of melatonin becomes more evident. As an example, in the most recently published Poison Control data from 2021, there were 54,669 single ingredient exposures reported and 44,868 were in children 5 years of age and under. Although these numbers sound daunting, the medical outcomes for all reported melatonin exposures including those involving adults reveal that the vast majority of outcomes were minor in nature (9,449) or reported no adverse effects at all (up to 82.7%). If you combine the minor effects with the no effect outcomes, they account for 99.7% of all exposures,” said Kingston. “When looking at melatonin, the exposure numbers are in favor of demonstrating safety, not unreason risk or injury.”
Category leader
Melatonin dominates the sleep supplements category and is responsible for over 90% of all sleep supplement sales in natural and mass retail channels, according to data from SPINS.
Consumers are also taking it in higher doses, with a 2022 SleepFoundation.org survey placing the average dose at 4.8 milligrams.
Maria J. Monagas, PhD, Principal Scientist- Dietary Supplements and Herbal Medicines, United States Pharmacopeia, said that while USP has monographs for melatonin (ingredient) and melatonin tablets, it does not have a monograph for melatonin gummies yet.
“For developing this monograph, we welcome data from manufacturers, including: Technical specifications, master formula, analytical methods, validation report and stability studies,” offered Monagas.
Industry goes into defense mode
The letter has been met with pushback from several trade groups who attacked the study’s methodology and note melatonin’s long history of use.
The Council for Responsible Nutrition doubled down on its criticisms, issuing a statement and a video which pointed out that the product samples used were products marketed for adults.
“Almost all of the 25 products sampled contained adult servings and are expressly labeled for use in adults, yet the authors conflate their findings with pediatric data. More concerning, they erroneously applied the FDA’s drug standard for overage/underage of ingredients (+/- 10 percent) for ingredient levels. The FDA’s regulation of dietary supplements requires companies to ensure that the products they sell meet 100% of label claim throughout the product’s shelf life. To do this, companies determine an appropriate and safe amount of ingredient overage that would provide at least the labeled per serving amount of each ingredient over time as degradation naturally occurs. This practice is permitted by FDA regulations,” the statement said.
“Supplement companies go to great lengths to ensure their products contain safe and consistent levels of dietary ingredients, as labeled,” said Steve Mister, President and CEO, CRN. “And while there may be some variability in overages as companies adhere to the FDA’s requirements regarding shelf life and potency, it does not mean there is a risk in taking these products as intended. It’s a misleading comparison to look at scenarios where kids, for example, got their hands on an entire bottle of adult gummies and became ill after eating multiple servings, versus having slightly more of an ingredient in a single serving that, if taken as directed, would pose no harm.”
“This report does a complete disservice to a safe product when it is used according to manufacturer’s instructions,” added Steve Mister. “Parents know how to take care of their own kids, and, often in consultation with their health care providers, have been safely giving the pediatric versions of these melatonin products to their children for years.”
Consumer Healthcare Products Association also issued a response, stating that melatonin is a safe and beneficial dietary supplement for adults and children, regulated by the FDA, and consumed by millions of Americans.
“FDA regulations require dietary supplements to contain a minimum of 100% of the amount claimed on the product label throughout its shelf life. Intentional ingredient overage is permitted for nutrient longevity and safe for consumers when within known safety levels consistent with good manufacturing practices (GMPs),” CHPA added.
“Use caution”
While the larger findings underscore the concern over the quality and safety, formulation experts said this letter also highlights the challenges of gummy manufacturing. According to Mark Miller, PhD, president of Kaiviti Consulting, gummy manufacturers often anticipate that heat will destroy melatonin levels and therefore add excessive overages.
He called the new letter “Worrying, but not surprising.”
“Making gummies exposes the ingredients to high temperatures that can break down the structural integrity of the ingredient. In the case of melatonin, it could limit the amount of melatonin that is present. If the manufacturer tries to control this with high overages then there is the possibility of getting too much. Gummies are not the easiest of vehicles for manufacturing, the constraints may not be well suited to melatonin. Bottom line: it’s a hormone–not a botanical extract, or a vitamin, or a mineral,” said Miller. “I suggest caution.”
Crystal Webber, founder of Niche Nutrition, said a couple things in the letter stood out to her.
“Based on assumptions that the analytical testing completed for the study was able to currently extrapolate melatonin from the gummy matrices and confirmed by a re-test, what I’m seeing here is a combination of two separate (but still serious) issues; one being children getting access to adult products that contain melatonin doses too high for children even at label claim, and the other being the concerning number of adult melatonin gummies that contain an amount of melatonin far above and beyond what would be acceptable for a conservative overage intended to maintain shelf life.”
Webber, who is a consumable product formulation specialist, said melatonin and other small-dose nutrients can be challenging to properly formulate when working with a complicated dose form such as a gummy.
“In addition to consistently completing analytical testing on incoming raw materials and every finished product gummy lot (preferably from a variety of samples taken from the beginning, middle, and end of the batch), manufacturers must adjust the formulation and processing steps to ensure consistent distribution of raw materials throughout the lot. I love seeing more and more companies offering consumers a copy of these test results on the website or upon request–that transparency can go a long way to foster renewed trust in dietary supplements.”
Source: JAMA
JAMA. 2023;329(16):1401-1402. doi:10.1001/jama.2023.2296
“Quantity of Melatonin and CBD in Melatonin Gummies Sold in the US”
Authors: P. Cohen et al.




