The foundation of Sundots began with Tolles’ co-founder, Harvard Medical School researcher Emilia Javorksi, M.D., M.P.H., who completed her post doctorate research at The Wellmen Center for Photomedicine.
“While she was there she identified a body of evidence around polypodium leucotomos,” Tolles told NutraIngredients-USA.
According to Tolles, there are at least 19 human studies – including one in The Journal of Clinical and Aesthetic Dermatology and another published in The Journal of American Academy of Dermatology – covering the efficacy of Polypodium leucotomos and its anti-oxidative effect on skin radiation damage.
“From a clinician research perspective, there’s real quality evidence that this could be a great consumer product,” Tolles said.
Tolles and Dr Javorski launched Sundots in March through the crowdfunding site Indiegogo, raising $190,000. Sundots gummies is sold direct-to-consumer for a one-time purchase of $50 per bottle or a monthly delivery of $29 per bottle.
‘Toolbox approach’ to sun protection
Sun protection, specifically dietary supplement makers advertising sun protection claims, were called out by FDA commissioner Scott Gottlieb in a public statement earlier this year admonishing consumers about the dangers of “products purporting to provide protection from the sun that aren’t delivering the advertised benefits…and that are misleading consumers, and putting people at risk.”
The FDA sent out warning letters to those companies illegally marketing pills and capsules labeled as dietary supplements that make unproven drug claims about protecting consumers from sun exposure harms without meeting the FDA’s standards for safety and effectiveness.
Tolles emphasized that Sundots is in no way claiming to be a replacement to sunscreen and UPF protected clothing, but instead believes the sun protection industry is lagging behind in effective solutions for getting more people to protect their skin effectively on a daily basis.
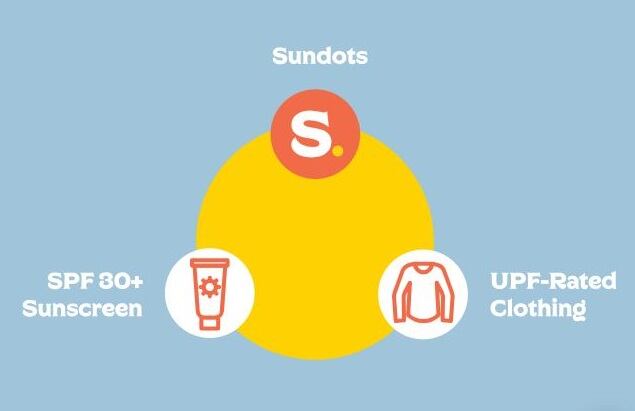
A three-pronged approach (sunscreen, Sundots, and UPF rated clothing) has been central to the brand’s messaging since day one, according to Tolles.
“The best sun protection is always going to be a combination of Sundots, sunscreen, and UPF rated clothing.
“We know that people are asking for better sun protection solutions which is why we know our approach of polypodium as an adjunct from the inside is a really great complement to what we know can already be effective topically with sunscreen and additional for UPF rated clothing.”
Compelling consumers to do all three steps to sun protection is not an easy task, Tolles acknowledged, which is why the convenience of taking Sundots is so important to its brand messaging and digital communications.
“We talk a lot about the user experience of sunscreen and that leaves a lot to be desired. It’s because we have a lot of competing demands on our time. The convenience of the gummy is so important – I’d rather give them better solutions than just the wagging of the finger,” Tolles added.
“We also know that it’s going to take way more than our first product to solve this problem, because it is so big and deep.”
Retail sales approach
While getting on the store shelves of a major drug store retailer such as CVS would be a huge coup for many other brands, Tolles said the brand is still figuring out its go-to-market strategy.
“We don’t’ really know where it belongs. If I had to guess, we’re with the sun protection products for the summer and the beauty products for the winter,” Tolles said.
“The bigger issue with a drug store approach is that no one’s asking for ‘photo-oral protection’ in these stores right now. Walgreens is not where you go to discover new products so I would expect that we would focus more on partnerships like a QVC or a goop (Gwenyth Paltrow’s beauty and wellness site) for example to really help us tell the story alongside the product.”