The trial found little reduction in 'bacteriuria plus pyuria' benefit among nursing home-dwelling retirees with an average age of 86 years taking two daily cranberry supplements each containing 36 mg of the active constituent proanthocyanidins (PACs) – equivalent to about 600 ml of standard cranberry juice.
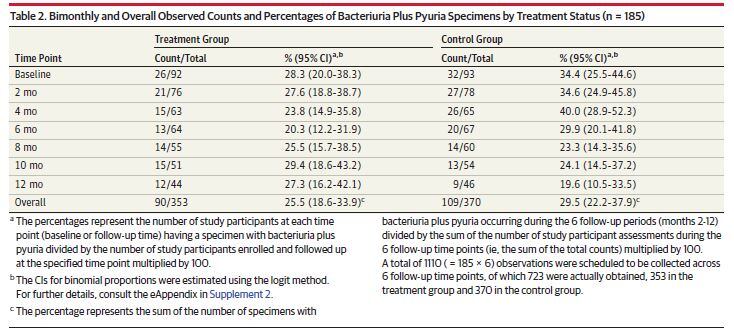
Of the 147 women who completed the 1-year study (out of 185 who began - 33 died and some became incontinent from the initial selection) the researchers, led by Dr Manisha Juthani-Mehta from the Yale University School of Medicine, found “no significant difference in presence of bacteriuria plus pyuria over 1 year” in the treatment group.
Although the research focused on bacteriuria plus pyuria - which is said to effect 25-50% of women in nursing homes - the Journal of the American Medical Association (JAMA) also published a commentary by Dr Lindsay E. Nicolle, from the Department of Internal Medicine at the University of Manitoba in Winnipeg, Canada.
She highlighted other studies with negligible UTI results when a precise “definition for symptomatic infection was applied”, equating bacteriuria plus pyuria with UTI in the process, to the chagrin of some cranberry researchers and the cranberry sector.
Dr Nicolle said the promotion of cranberry for prevention of UTI via the “popular press or online advice” was beyond “rational reasoning” and instead backed further research in medical avenues like antibiotics and immunity drugs.
“…cranberry products should not be recommended as a medical intervention for the prevention of UTI,” she wrote.
Cranberry & UTI
In the UK the National Health Service (NHS) advises that “Drinking cranberry juice has traditionally been recommended as a way of reducing your chances of getting cystitis. However, large studies have suggested it doesn't make a significant difference.”
In the EU cranberry products are approved as medical devices and able to make UTI treatment claims although this regulation is under review. Cranberry products are not approved under the EU nutrition and health claims regulation (NHCR) for prevention claims despite multiple submissions from various ingredient suppliers and manufacturers.
The Massachusetts-based Cranberry Institute – established in 1951 and which counts global cranberry ingredients and juice maker Ocean Spray as a member – slammed the study for choosing an irrelevant population on which to test cranberry PACs effectiveness.
The Institute said the researchers therefore failed to assess cranberry UTI efficacy “as they treated individuals that did not suffer from UTIs or persistent bacteriuria plus pyuria.”

Dr Juthani-Mehta noted about a third of the women had bacteriuria and pyuria present at enrolment, and that there had been a significant reduction in the first six months before stabilising at initial levels in the subsequent six months. But the Cranberry Institute likened the study to “evaluating the effectiveness of a weight loss program on individuals with normal body weight – the absence of weight loss will not truly reflect the efficacy of the program.”
The Institute said the editorial by Dr Nicolle was “misleading and suffers from fatal flaws.”
Rather than being “time to move on from cranberries” as Dr Nicolle suggested, the Institute suggested more cranberry research was required to “attenuate the use of antibiotics and prevent this pervasive condition.”
Cranberry defence
Gunter Haesaerts, owner of French cranberry supplements manufacturer Pharmatoka, which supplied the trial with supplements, issued a letter to Pharmatoka ‘distributors and suppliers’ that criticised the researchers for changing the study aims after asking to use his firm’s ‘ellura’ and ‘urell’ cranberry products in the study when it was conceived in 2012.

“…we wish to point out that the initial submission for this trial, validated (and funded) by NIH on April 20, 2012, was titled ‘cranberry capsules for the prevention of urinary tract infection in nursing home patients’,” Haesaerts wrote.
“It now appears that the study title was changed to focus on asymptomatic bacteriuria and pyuria presence as the primary objectives. In that context and with this specific population of elderly women in nursery homes, the negative results do not come as a surprise.”
Haesaerts was even more critical of the editorial written by Dr Nicolle.
“Her objective was not so much to comment on the study itself but rather impose her own biased conclusions and recommendations on her fellow practitioners.
“It reeks of heavy lobbying in favour of the use of antibiotics and aims at eradicating proven cranberry based prophylaxis against recurrent UTIs.”
‘Baffled’
Amy B. Howell, PhD, associate research scientist at the Marucci Center for Blueberry Cranberry Research at Rutgers University in New Jersey, said cranberry research had improved since a critical Cochrane review in 2012, and agreed with Haesaerts that the endpoint was not suitable for cranberry and had already “exhibited inconclusive results in past studies.”

“Bacteriuria, which was the actual focus of this study, occurs frequently in elderly nursing home patients and is not necessarily an indicator of clinical UTI.”
She said it was “frankly very disappointing” that a journal like JAMA hadn’t consulted cranberry researchers before publishing its editorial; researchers “familiar with some of the research caveats that have influenced the outcomes of previous studies.”
Dr Howell was “baffled” JAMA would promote antibiotic use when anti-microbial resistance rates were rising and cranberry “has shown in decades of research to be a promising and effective alternative prophylaxis.”
The study was sponsored by the Claude D. Pepper Older Americans Independence Center and the National Institute on Aging and National Institutes of Health (NIH).
Recurrent UTI?
Dan Souza, VP of sales & marketing at cranberry supplier Naturex-DBS, went deeper into what constituted a UTI, or more precisely a ‘recuurent’ UTI.

He referenced an Canadian Urological Association Journal study from 2011 that found 'A threshold of 3 UTIs in 12 months is used to signify recurrent UTI.'
“In this present study only five subjects were recurrent UTI suffers at baseline - one in the treatment group, four in the control group," Souza said. “Since less than 4% of the population suffered from recurrent UTI in the previous year, the study population was not suitable for a reduction in UTI outcome.”
He also questioned the study's focus on PACs to the exclusion of other molecules active in cranberries.
Source:
[Study] Journal of the American Medical Association (JAMA)
Published online October 27, 2016. doi:10.1001/jama.2016.16141
‘Effect of Cranberry Capsules on Bacteriuria Plus Pyuria Among Older Women in Nursing Homes: A Randomized Clinical Trial.’
Authors: Juthani-Mehta M, Van Ness PH, Bianco L, Rink A, Rubeck S, Ginter S, Argraves S, Charpentier P, Acampora D, Trentalange M, Quagliarello V, Peduzzi P.
[Editorial] JAMA
Published online October 27, 2016. doi:10.1001/jama.2016.16140
‘Cranberry for Prevention of Urinary Tract Infection?Time to Move On’
Author: Lindsay E. Nicolle
