4-mei - an impurity generated in the production of some caramel colors produced using ammonium compounds - hit the headlines again in January after Consumer Reports published test results showing what it claimed to be “concerning” levels of 4-mei in soft drinks and called on the FDA to set federal limits well below those enshrined in California’s Proposition 65 list.
The FDA agreed to conduct new tests, but said at the time that it had no reason to believe that 4-mei from caramel colors posed a health risk (click HERE). Speaking to FoodNavigator-USA on August 15 after presenting a poster at the American Chemical Society annual meeting, the agency reiterated its earlier comment: "We have no reason to believe that at current levels it poses a health concern."
FDA is currently evaluating the carcinogenic potential of 4-mei
However, tests and analysis are ongoing and stakeholders should not try to pre-judge the agency's response to citizen's petitions on 4-mei, said a spokesperson on August 22:
"FDA presented its refined exposure assessment in a poster session at the American Chemical Society meeting in San Francisco, California. FDA will use these 4-mei exposure estimates in conjunction with review of the available toxicological data for 4-mei in order to address the citizen petitions before the Agency.
"The World Health Organization’s International Agency for Research on Cancer (IARC) evaluated 4-mei and determined it to be a Group 2B, possibly carcinogenic to humans. FDA is currently evaluating the carcinogenic potential of 4-mei. These efforts will inform the FDA’s safety analysis, and will help the agency determine what, if any, regulatory action needs to be taken."
Intakes of 4-mei from carbonated soft drinks have fallen recently owing to wider adoption of low-4-mei caramel color variants
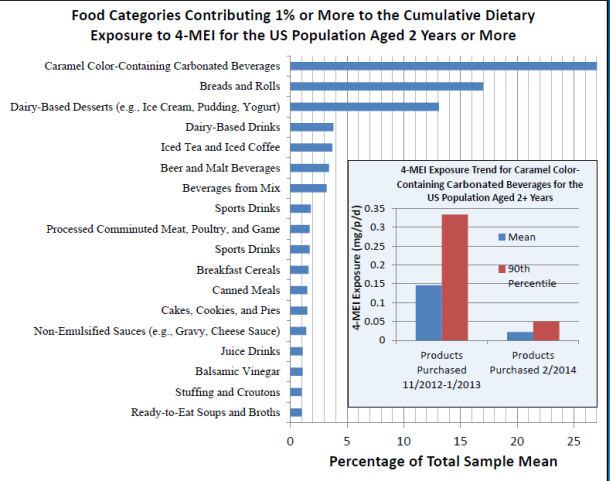
In its poster, the FDA said 400 representative products had recently been sent to a contract laboratory to assess 4-mei levels, while it had also done testing in-house.
The results were then correlated with dietary intake data from the National Health and Nutrition Examination Survey (NHANES) to determine estimated exposure levels.

As expected, when it came to 4-mei levels from caramel colors, colas were the biggest contributors, although levels have come down recently as more players have switched to using low 4-mei caramel colors, said the FDA.
“FDA will use the 4-mei exposure estimates in conjunction with review of the available toxicological data for 4-mei in order to address the citizen petitions before the Agency."
A 2011 petition urged the FDA to ban class III & IV caramel colors, while a 2014 petition asked it to set a maximum threshold level for 4-mei in caramel colors, identify caramel color classes on ingredients lists and bar products made with caramel colors from making ‘natural’ claims.
Click HERE to download the FDA's poster presentation on 4-mei.