Berberine supplement may help manage blood sugar levels for prediabetics: RCT

Data from a randomized, double-blinded, placebo-controlled pilot trial published in BMC Endocrine Disorders indicated that 1,500 mg per day of the branded Himaberb ingredient produced by Gramen Botanicals led to a reduction in blood sugar levels to “below prediabetic thresholds."
“These results are clinically meaningful and have the potential to impact the management of patients that are classified as prediabetic,” the researchers wrote. “Control of blood glucose in individuals with prediabetes to below clinical thresholds using natural and non-toxic agents, as demonstrated here, may benefit disease outcomes and safety for patients.”
Berberine
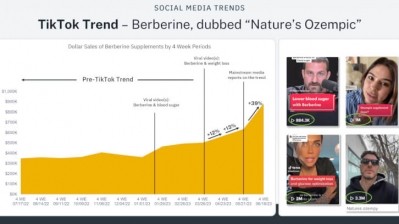
Sales of berberine supplements spiked in the U.S. in 2023 after TikTokers linked the compound’s potential glycemic control effects to those of Ozempic, Novo Nordisk’s prescription drug for diabetes. The drug also produces significant weight loss and led some influencers to refer to berberine as #naturesozempic.
As a result of the TikTok craze, one leading brand told NutraIngredients-USA that sales increased 623% before the company ran out of stock.
Berberine is a natural alkaloid found in several plants, including goldenseal (Hydrastis canadensis), Indian barberry (Berberis aristata), and Oregon grape (Berberis aquifolium). Meta-analyses support berberine’s glucose support potential, with the data also showing improvements in BMI (for example: Guo et al. 2021, Oxid Med Cell Longev).
The majority of the studies to date have focused on people with diabetes, and the new study is reportedly one of the first to examine the botanical’s potential benefits in people with prediabetes.
Study details
Thirty-four people between the ages of 18 and 55 with prediabetes, as defined by the American Diabetes Association, were randomly assigned to receive either 500 mg of Himaberb or placebo three times per day for 12 weeks.
Himaberb is described as an aqueous extract of Berberis aristata root, standardized to 97% purity by HPLC, manufactured as per Japanese Pharmacopoeia (JP) and encapsulated in 500 mg doses.
Results of the study showed that fasting plasma glucose (FPG) levels decreased by an average of 21% in the berberine group after 12 weeks, compared to the control group. In addition, fasting insulin decreased by almost 20%.
HbA1c (a measure of how well controlled the blood sugar is) decreased by 15%, while HOMA-IR (a measure of insulin resistance) fell by 33%.
The 12 week improvements in FPG and HbA1c led to a return of those markers to “below the clinically defined thresholds for prediabetes,” the researchers reported.
“Given its potential insulin sensitizing effects […], berberine may be useful in intervention for early stages of insulin resistance in patients who have not been diagnosed with diabetes,” they wrote. “In addition, given its favorable safety profile and evidence of efficacy, berberine may be an attractive supplemental therapy for glycemic control compared to other supplemental drugs with less conclusive evidence for safety and efficacy, such as chromium, magnesium, nicotinamide or vanadium.”
The researchers noted that their study was small and limited to only 12 weeks of intervention. They called for a long-term multicentric study with a larger number of participants to “provide more robust data that is generalizable to the broader population”.
Safety & efficacy
Commenting on the study’s findings, Samarth Vikram Singh, CEO of Gramen Botanicals, the manufacturer of Himaberb, stated: “We procure a sustainable source of wildcrafted roots of the Berberis aristata plant to manufacture Himaberb. This clinical trial demonstrates Himaberb’s efficacy and safety over a three-month period.”
Source: BMC Endocrine Disorders
23, 190, doi: 10.1186/s12902-023-01442-y
“Efficacy and safety of HIMABERB Berberine on glycemic control in patients with prediabetes: double-blind, placebo-controlled, and randomized pilot trial”
Authors: A. Panigrahi, et al.