FDA issues warning letters on tianeptine 'supplements' making opioid-related claims

Tianeptine is an anti-depression drug that is not approved in the US for medical use, although it is approved in Europe, Asia, and Latin America. According to the US Centers for Disease Control and Prevention (CDC), the misuse of tianeptine in the US is increasing for its opioid-like effects.
“The FDA is aware of serious adverse events that are associated with tianeptine. Consumers may inadvertently find themselves addicted to tianeptine and should avoid all products containing this ingredient, especially those claiming to treat opioid use disorder (OUD),” states the FDA in its constituent update.
“In addition to potential adverse events, reliance on products with unsubstantiated claims may delay those who suffer from OUD from entering recovery and may put them at greater risk of overdose and death. We know that patients receiving FDA-approved medication-assisted treatment (MAT) cut their risk of death in half, according to the Substance Abuse and Mental Health Services Administration.”
The ingredient is now allegedly turning up in products labeled as “dietary supplements”, prompting the FDA to issue warning letters this week.
Because tianeptine does not qualify as a dietary ingredient, is not an approved food additive, and is not GRAS, dietary supplements containing tianeptine are adulterated under the Federal Food, Drug, and Cosmetic Act, states the Agency in letters to Henderson, NV-based MA Labs LLC and Ridgeland, MS-based Jack B Goods Outlet Store.
“In addition, the products that were the subject of these Warning Letters are considered to be drugs under the Act because they are marketed with claims to cure, mitigate, treat or prevent a disease, including opioid use disorder (OUD). These products are not approved for such uses,” added FDA.
Gottlieb: "We won’t stand by and allow this to happen"

Scott Gottlieb, MD, FDA Commissioner, stated: “This action is part of a broader effort we have underway to re-examine our resources and authorities related to products marketed as dietary supplements, and outline a new policy on how we intend to more vigorously fulfill our obligations to protect consumers from dangerous products and unlawful claims. We’ll have more to say on our policy efforts very soon.
"The bottom line is this: we’ve seen growing instances where profiteers are pushing potentially dangerous compounds – often with unproven drug claims and crossing the line when it comes to what defines a dietary supplement. These potentially illegal activities put the entire dietary supplement industry at risk by confusing consumers, harming patients and tainting good dietary supplement products by associating them with the activities of bad actors.
“In this case, these companies are preying on vulnerable patients who may be seeking alternative treatments to serious medical conditions like opioid use disorder. They’re also selling products with known safety issues. We won’t stand by and allow this to happen. These warning letters are one part of our enforcement plan and we’ll continue to take action to protect public health.”
Claims
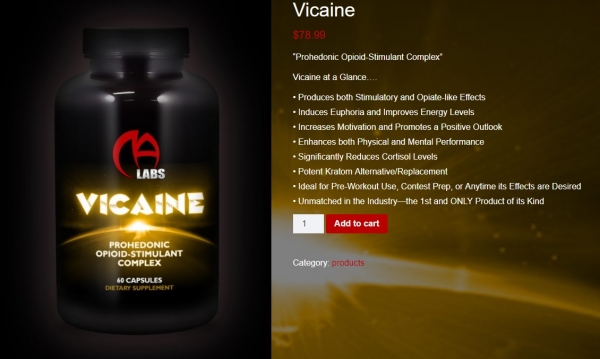
Among the claims allegedly made by MA Labs on its Vicaine product and cited by the FDA are:
“[P]ain-killer, … euphoriant, speed-ball in a bottle, stress-reducing agent…”
“Vicaine possesses both stimulatory and opiate-like properties with a strong dopaminergic element…”
“Containing potent dopaminergic-stimulants and a full-opioid agonist…”
Alleged claims made by the products Tianaa Red, Tianaa White, and Tianaa Green listed on the Jack B Goods Outlet Store website included:
“It has been our experience that there is a natural reaction that affects the serotonin receptor site providing an unparalleled solution to cravings for opiates. Kratom initially filled this need in providing mental clarity and energy without the crash. Now, these four alternatives can replicate the benefits of Kratom with perfection.”
“There has never been such a clear choice for pain and anxiety.”
Both MA Labs and Jack B Goods Outlet Store were contacted via email for comment by NutraIngredients-USA, but neither responded prior to publication.
The Tianaa products on the Jack B Goods Outlet Store website were listed as “out of stock” and the website noted for each: “The description of this product is under review and this product can not be purchased through our website”.
The Vicaine product was still be available for purchase from https://masupps.com/product/vicaine/ as of 8am Central time on November 21, 2018 (see screenshot below).
FDA approach isn’t “whack-a-mole”
Commenting on the FDA’s announcement and warning letters, Dan Fabricant, PhD, CEO of the Natural Products Association (NPA), told us that this shows that the agency’s approach is not ‘whack-a-mole’ and FDA is clearly focusing on area where the most problems are.
Duffy MacKay, ND, Sr VP of scientific and regulatory affairs for the Council for Responsible Nutrition (CRN), applauded the FDA’s actions, adding that CRN is aligned with FDA around protecting consumers from illegal products falsely identified and marketed as dietary supplements, particularly around the very serious issue of opioid use disorder.
“We have a heightened sensitivity around this issue,” said Dr MacKay, “and our industry has to be disciplined in supporting the agency in these actions.”
Indeed, CRN and NPA, along with the American Herbal Products Association (AHPA), the Consumer Healthcare Products Association (CHPA), and the United Natural Products Alliance (UNPA) issued a joint statement in December 2017 reminding consumers, retailers, and product marketers that dietary supplements cannot claim to treat opioid addiction.
“Consumer safety and access to safe products are important to both CRN and the FDA. CRN recommends that consumers seeking treatment of an opioid use disorder or addiction talk to a qualified healthcare professional or a public health authority,” added Dr MacKay.
Earlier this year, the FDA and FTC announced a crackdown on companies marketing dietary supplements to consumers trying to overcome opioid addictions. The FDA issued 11 warning letters to dietary supplement companies, while the FTC issued an additional four warning letters. According to an FTC statement, the companies were warned about “illegally marketing products with unproven claims about their ability to help in the treatment of opioid addiction and withdrawal.”