Overall FDA's 483 observations are down, but basic GMP failures still rampant, consultant finds
Overall, Boardley said 483 observations arising from FDA inspections of food facilities (which includes dietary supplements) declined from a high of about 2,200 in fiscal year 2013 to about 1,500 in FY 2014. Boardley cautioned that a key piece of data is yet to be reported, which is the overall number of inspections. Without that, it’s hard to say if the situation is actually improving, but she did say that she believes that the numbers as they stand represent a real decline.
Basic failures still rampant
While that would seem to be good news, Boardley said delving deeper into the data shows a disturbing trend. Basic failures on GMP compliance still make up the largest slice of the overall pie, and the size of the piece is growing.
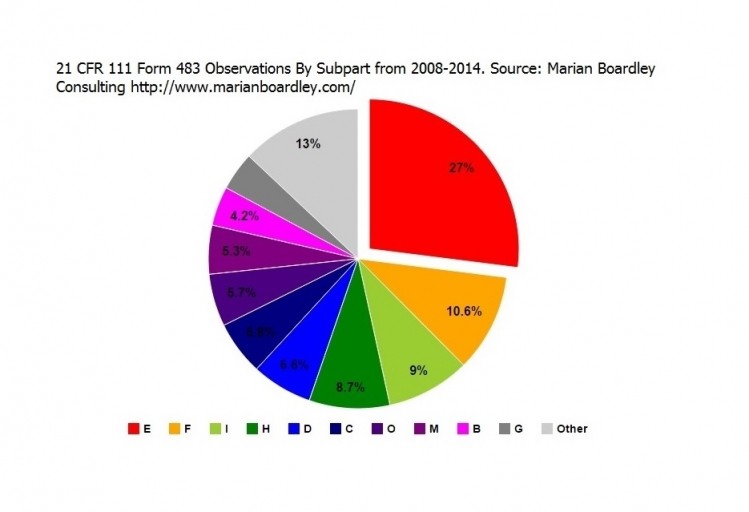
“Observations under subpart E continue to go up,” Boardley told NutraIngredients-USA. “As a percentage of overall observations, they were 29% last year and 30% this year. That is the case that a company hasn’t set a specification for a finished product or an incoming component or ingredient. When FDA inspects a facility and they don’t find specifications in place, that is a pretty fundamental GMP failure.”
That sentiment was echoed by Daniel Fabricant, PhD, CEO of the Natural Products Association. Until late April, Fabricant was the director of FDA’s Division of Dietary Supplement Programs.
“I think what matters is, what are the particular observations? Are we seeing observations where people simply didn’t do something they are supposed to do, or are those observations of a more technical nature?” Fabricant said.
“Not having specifications, that is an observation that is still at the core of GMPs,” he said. “If we get to a point where FDA says, they’ve inspected every facility, and they come back through and a firm that was cited a year or two ago for not having specifications now has them but FDA disagrees with their protocol, that would be a sign of progress. We want to get to where we are seeing more of those sort of technical observations.”
Other emphases
Boardley said that in addition to the large number of basic GMP shortcoming in the way of missing specifications, the data shows that the agency is also looking more closely at how companies deal with product complaints. Or don’t. The observations for subpart O came in at 5th place with 96 observations, or 6.2% of the total number of observations. In No.1 place stand the aforementioned part E observations, dealing with process control and specifications. Inspectors cited firms 470 times for these deficiencies, or 30.2% of the total. Following that subpart in 2nd, 3rd and 4th place were quality control (subpart F, 160 observations, 10.3% of the total), batch records (subpart I, 157/10.1%) and master records (subpart H, 151/9.7%). Boardley offers a number of charts on her website that summarize the data.
Also mentioned were observations on deficiencies relating to adverse events reports, for which firms were cited six times. Boardley said this pointed out a weak point of her process, namely that she has to rely on how the inspectors themselves code their observations. An inspection citation about a complaint might fall under adverse events depending on the nature of the incident, but that depends again on the inspector, she said. Similarly, there were 8 observations about “records” which could fall into any number of categories. This is true of other categories of observations, too.
Boardley said her ultimate goal for combing through the data is to help her clients manage risk.
“That’s the only way to really assess your risk, to see where FDA is placing its emphasis and to see how that compares with how you are doing things,” Boardley said.









