Kemin’s ZeaONE zeaxanthin supplement approved for Brazilian markets

Kemin announced that ANVISA, Brazil’s national Health Surveillance Agency, has approved the sale of its product, ZeaONE™ Zeaxanthin. This approval allows Kemin to immediately begin marketing the product in Brazil. “ZeaONE is a dietary free-form zeaxanthin ingredient naturally sourced from marigolds produced by Kemin,” the company said in a press release.
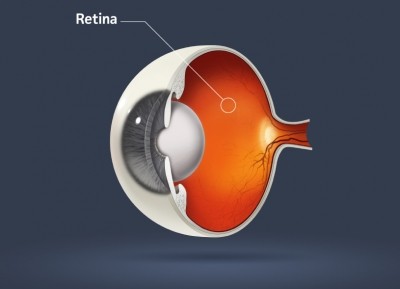
Accoridng to Kemin, zeaxanthin, like lutein, is “proven to act as a protective antioxidant and filter of blue light in the eye and skin.” Other benefits Kemin said comes from daily zeaxanthin supplementation includes skin health, improvement in visual performance, and a reduction of risk of certain eye conditions.
“The latest findings from the U.S. National Eye Institute’s second age-related eye disease study (AREDS2) reports that 2 mg of zeaxanthin, along with 10mg of FloraGLO Lutein, can slow the progression of age-related macular degeneration,” the release said.
The zeaxanthin in ZeaONE comes from a patented line of marigolds exclusively licensed to Kemin and naturally containing the high levels of zeaxanthin (3R, 3’R zeaxanthin) found in the fruits and vegetables in the human diet.
Kemin claimed that its patent-protected marigold seeds were produced with “good agricultural practices [and] tightly controlled supply-chain management.”
ZeaONE is commercially available under DSM’s brand OPTISHARP® Natural.